Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Advances in reperfusion therapy have led to the development of a large population of patients with chronic heart failure. Modulation of cardiac tissue is the next great frontier to lessen symptoms of heart failure and improve patient outcomes.
In general, the field has failed to demonstrate robust results in large scale studies. The time for small studies with multiple subgroups is past.
Improvements in cardiac function with cell therapy do not necessarily imply improvements in electrical conduction or arrhythmogenic risk.
Multiple delivery strategies and devices have entered the clinical realm.
The generation of cardiac myocytes from embryonic stem cells and induced pluripotent stem cells suggests autologous cardiac myocyte regeneration may be possible in the future.
Recent clinical data suggest that autologous cell therapy may be more efficacious than allogeneic cell therapy for heart failure.
There is growing interest in strategies to modulate stem cells to enhance the overall benefits of stem cell therapies.
There is greater understanding of the molecular mechanisms associated with regenerative therapies leading to novel treatment strategies.
There is growing clinical evidence concerning the use of stem cells to treat chronic chest pain.
The many efforts to maximize therapy for patients with acute myocardial infarction (AMI) have yielded significant benefits. Beginning first with thrombolytic therapy for AMI, and more recently with the wide availability of primary percutaneous coronary intervention (PCI) for ST-elevation myocardial infarction (STEMI), the mortality rates of this devastating ischemic event has decreased from almost 15% in clinical trials in the late 1980s to less than 5% in recent primary PCI trials. Prior to these advances, ischemic heart disease was the leading cause of chronic heart failure (CHF). Although further improvements in reperfusion are needed, the current advances have led to the growing epidemic of CHF, with many patients surviving what in the past might have been fatal events.
With these advances, the prevalence of congestive heart failure has increased dramatically over the preceding decade, with currently more than 10% of the United States population older than 65 years of age carrying the diagnosis. Although the mechanisms are still under investigation, the development of CHF following myocardial infarction is more than just the loss of contractile tissue; it is also partly determined by the ventricular remodeling that occurs in response to myocardial necrosis. The inflammatory response to myocardial necrosis leads to infarct expansion, dilatation of the left ventricle (LV) cavity, and replacement of cardiomyocytes with fibrous tissue. Furthermore, the remodeling process results in an infarct border zone that is ischemic and may drive inflammation and further adverse remodeling. Treatment of this border zone is at least one target of cardiovascular regenerative therapies because increasing the contractile reserve of the border zone is correlated with improved ventricular remodeling. Currently available therapies to alter the remodeling process and the progression to CHF remain limited, and death rates from CHF continue to rise. Based on current trends, the problem is predicted to increase to greater than 6 million people by the year 2030.
The increasing burden of CHF has been addressed with pharmacologic therapy, which in some can delay and improve the morbidity and mortality of CHF; electrical therapy including cardiac resynchronization therapy; left ventricular remodeling surgery; and mechanical therapy, with the recent approval of the first left ventricular assist device for destination therapy. In terms of pharmacologic therapy, the noteworthy PARADIGM-HF trial (a multicenter, randomized study to evaluate the efficacy and safety of LCZ696 compared to Enalapril on morbidity and mortality in patients with CHF and reduced ejection fraction) demonstrated a long-sought benefit for the combination of angiotensin-neprilysin inhibition with sacubitril-valsartan. This combination of high-dose valsartan with neprilysin inhibition was found to be superior than enalapril in reducing the risk of death and heart failure hospitalizations. Whether these greater benefits will be seen in clinical benefit or will be seen with additional pharmacologic strategies is of great debate.
To address the increasing prevalence of CHF, over the past 15 years cardiovascular medicine has taken great interest in the potential of regenerative therapies; more specifically, in stem cell therapies to prevent and treat cardiac dysfunction, since this approach, unlike all others, has the opportunity to address the underlying problem—loss of cardiac myocytes due to ischemic death. Although there was much excitement and fanfare in the beginning, the field has found the need to address many challenges. That said, there is progress and hope to improve patient outcomes in the future.
The field of cell transplantation for the treatment of left ventricular dysfunction following ischemic injury continues to make significant progress, currently with dozens of studies completed and meta-analyses that continue to suggest overall benefit to those patients treated compared with controls. The goal has not changed—define a cell type that possesses the capacity to incorporate into the recipient myocardium and that will be able to survive, mature, and electromechanically couple to each other and to native cardiac myocytes.
The field began with differentiated cell transplantation ( Fig. 61.1A ); the goal was to functionally achieve myoplasty through the transplantation of autologous skeletal myoblasts that would engraft into injured myocardium in patients with chronic ischemic heart failure. Another strategy that has been implemented in clinical studies is stem cell mobilization (see Fig. 61.1B ), which involves the pharmacologic mobilization of a patient’s own bone marrow cells into the blood stream, homing of these stem cells to engraft into areas of myocardial damage, and then directing the engrafted stem cells to differentiate into cardiac myocytes and vascular structures that fully integrate with the native myocardium. By far the most comprehensively studied strategy to date that has shown some benefits is stem cell transplantation (see Fig. 61.1C ) that involves the removal of stem cells from the bone marrow of the patient (autologous) or a well-screened donor (allogeneic) and then injection of the whole bone marrow or a selected population of bone marrow-derived stem cells into the infarct zone. Questions abound as to how these strategies will move forward and lead to clinical benefit in preventing (treatment in patients in the periinfarct period) or treating (treatment in patients with CHF) cardiac dysfunction. In this chapter we attempt to summarize the current state of knowledge, inform the reader of studies that are ongoing and what their findings may mean, and discuss where the field may be moving.
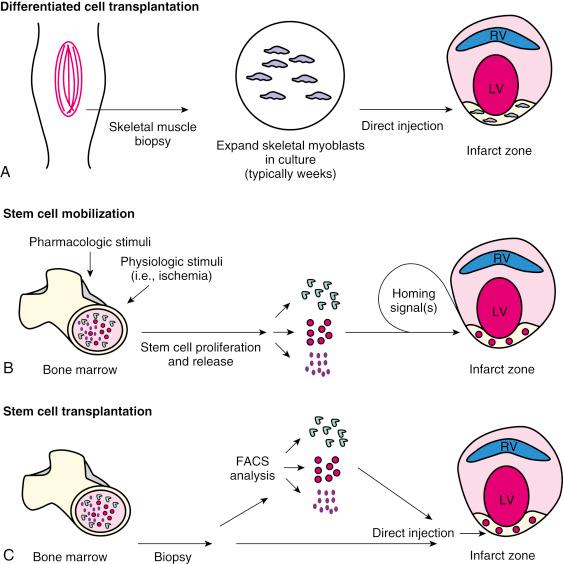
The goal of differentiated cell transplantation is the substitution of scarred myocardium with living viable cells ultimately leading to overall improvement of myocardial function. A number of cell types, including smooth muscle cells and skeletal myoblasts, have been studied as potential candidates for differentiated cell transplantation. The value of these cell types is born out of their accessibility and their ability to be expanded in vitro prior to transplantation.
Arguably the first strategy of cell therapy for CHF was cardiac myoplasty (cardiomyoplasty). During this procedure the heart was wrapped in skeletal muscle that was then paced to increase contractility of the weakened heart. It was recognized that there was engraftment of skeletal muscle in the epicardial layers of the heart which we currently recognize was the ingrowth and engraftment of skeletal myoblasts.
Following these observations, the early preclinical and clinical studies with skeletal myoblasts set the stage for the potential benefits associated with the delivery and engraftment of exogenous cells into the heart to prevent and treat cardiac dysfunction. We learned that exogenous cells could engraft into the heart and that the engraftment of skeletal myoblasts could lead to clinical benefit, including inducing reverse remodeling of the left ventricular remodeling. We further learned the importance of mechanical coupling of exogenous cells to the cellular milieu of the heart. Skeletal myoblasts do not integrate into the electrical syncytium of the myocardium and have been shown in animal studies to induce slow conduction, increase risk of reentrant rhythm, and increase premature ventricular contractions and ventricular tachycardia in patients.
Significant knowledge was gained through studies of skeletal myoblasts (SKMB); however, because of limitations due to arrhythmogenic potential and lack of vascular growth, SKMB for prevention and treatment has been appropriately largely abandoned.
Since the turn of the century, a significant body of literature has rewritten the once strong-held belief that the heart cannot repair itself. We have learned that following myocardial infarction in humans, there is a transient mobilization of stem cells and expression of stem cell homing factors that recruit these cells to the heart. Human female hearts transplanted into males were found to have cardiac myocytes and vascular structures that stained positive for the Y chromosome, suggesting that these cells originated from the stem cells of the transplant recipient. Unfortunately, these studies demonstrate that stem cell engraftment and differentiation into cardiac myocytes is an infrequent event (0.02% cardiac myocytes, 3.3% endothelial cells). However, these studies do suggest that the normal physiologic response to myocardial injury is mobilization of stem cells, “homing” of these cells to the damaged myocardium, and differentiation of at least some of these stem cells into cardiac myocytes. Furthermore, if this natural repair mechanism can be potentiated, clinically meaningful myocardial regeneration may be achievable.
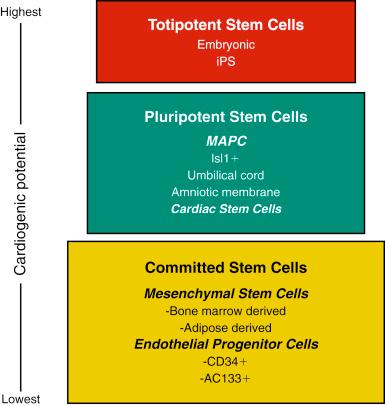
The excitement surrounding the use of stem cells is based on the unique biologic properties of these cells and their capacity to self-renew and regenerate tissue and organ systems. Fig. 61.2 groups different stem cell populations of interest in myocardial regeneration based on their cardiogenic potential. While once it was believed that all stem cell populations listed in Fig. 61.2 could differentiate into cardiac myocytes, it is currently quite clear that, although many of these cell populations may lead to improved cardiac function, virtually no adult stem cell can differentiate into cardiac myocytes. Arguably the one unmanipulated adult cell population that may hold the ability to differentiate into adult cardiac myocytes is cardiac stem cells (CSCs). All cells that have pluripotent to totipotent potential, such as embryonic stem cells (ESCs) and induced pluripotent stem cells (iPS), have been shown to generate adult cardiac myocytes.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here