Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Noninvasive ventilation may be used to increase airway pressure and support a failing respiratory system without the need for tracheal intubation or tracheostomy.
Intermittent positive pressure ventilation can be delivered by a variety of different techniques, many of which are coordinated with the patient’s own respiratory efforts.
Positive end-expiratory pressure increases the functional residual capacity, reduces airway resistance and may prevent or reverse lung collapse.
Any increase in mean intrathoracic pressure, as seen during positive pressure ventilation, impairs venous return and increases pulmonary vascular resistance, thus reducing cardiac output.
Artificial ventilation may damage the lung by exerting excessive pressures or volumes on lung tissue, or by causing repeated opening and closure of small airways with each breath.
A clinically useful artificial lung remains only a distant possibility, although extracorporeal systems that partially replace pulmonary gas exchange continue to evolve.
Chapter 27, Chapter 28, Chapter 29, Chapter 30, Chapter 31 outlined the numerous ways in which the respiratory system may fail to achieve its primary objective of gas exchange. This chapter describes the techniques available to clinicians to improve the gas exchange functions of the respiratory system, including supporting or replacing alveolar ventilation.
In many of the respiratory diseases described in Chapter 27, Chapter 28, Chapter 29, Chapter 30, Chapter 31 , the patient’s respiratory system is often treated with physiotherapy. This is more as part of long-term management, for example, in pulmonary rehabilitation programmes (page 333), but is also a useful part of treatment of acute lung problems, such as in ventilated patients and in the postoperative period. Despite extensive involvement of physiotherapists in respiratory care throughout the world, the evidence base for improved clinical outcomes as a direct result of physiotherapy interventions is weak. There are multiple possible reasons for this, not least of which are a lack of suitable studies, an underappreciation of the placebo effect and the part played by physiotherapy as a component of ‘care bundles’, which overall have beneficial effects.
The aims of respiratory physiotherapy can be broadly classified into the following three areas.
Physiotherapy can help to attenuate loss of lung volume due to atelectasis, consolidation or pleural effusion. Techniques include controlled mobilization, in which patients are assisted to exercise at a level which just increases their ventilation enough to generate deep breathing without causing anxiety or excessive fatigue, in other words, learning to achieve and maintain a state of ‘slight breathlessness’. Patient positioning is important for increasing lung volume (page 23) and ventilation/perfusion matching, and sitting upright is generally beneficial. Patients with pleural effusions or large intrathoracic tumours usually learn for themselves to lie with the nonaffected lung regions uppermost. Deep breathing exercises have many respiratory benefits, most of which relate to reexpansion and ventilation of dependent lung regions. Ten deep breaths per waking hour are recommended, including an end-inspiratory hold, if possible. Incentive spirometry may help patients achieve the most benefit from deep breathing. These devices provide visual feedback to patients about their inspiratory flow rate and volume, allowing these to be tailored to the optimal values for lung reexpansion in individual patients. Continuous positive airway pressure (CPAP) is used to increase lung volume and is described later. Finally, intermittent positive pressure breathing may be used, in which a positive pressure is applied to the patient’s airway during inspiration, followed by passive exhalation.
Dyspnoea can be treated by various techniques (page 320) and attempts made to alleviate patients’ perception of their breathlessness by teaching them specific techniques to control their breathing. Pulmonary rehabilitation (page 333) or noninvasive ventilation (see later discussion) are two techniques that can increase respiratory muscle efficiency or workload, respectively.
These techniques are used routinely in the care of patients with cystic fibrosis (page 335) and in other patients in whom airway secretions are increased or their clearance impaired, such as bronchiectasis or chronic obstructive pulmonary disease (COPD). Methods available include:
Humidification of airway gases, including ensuring the patient is systemically well hydrated.
Exercise, as previously described, which increases minute ventilation and thus expiratory flow rates. This increases the shear stress between gas flow and airway mucus, ‘pulling’ the mucus away from the airway (page 47). This then provokes coughing and expectoration.
Active breathing techniques, which includes active cycle of breathing, consisting of a combination of deep breaths followed by huffs, rapid exhalations through an open glottis and mouth. This helps to move mucus along the airway. The point in the deep breath where the huff is performed may influence where in the airway the maximal effect is achieved according to the site of the ‘choke point’ (page 47). Autogenic drainage involves performing controlled breaths with slow, large, inspirations, an inspiratory pause and then slow prolonged exhalation through pursed lips to maintain airway patency and prevent flow-related collapse (page 32). A series of these manoeuvres are performed at progressively increasing lung volume.
Postural drainage describes changing body position to allow gravity to contribute to drainage of secretions from specific lung regions. This is normally used in combination with the other breathing techniques described.
Manual techniques include percussion or vibration of the chest wall, usually combined with postural drainage. They aim to improve the clearance of mucus from the airway wall and may work by changing the physical properties of mucus during a cough, by improving airway-lining fluid and ciliary activity, increasing expiratory flow rate or freeing adhesive mucus from the airway wall. The optimal frequency of vibration is unknown, but animal studies suggest this is between 10 and 15 Hz.
Positive expiratory pressure (PEP) may be achieved by several devices, all of which impose an expiratory resistance, some of which also cause oscillations in expiratory pressure. Although PEP has been shown to prevent expiratory airway closure and reduce gas trapping, its effectiveness at improving clearance of secretions is uncertain.
Cough facilitation remains a key component of respiratory physiotherapy, particularly in neurological conditions when muscle function is inadequate. It may be achieved manually or mechanically.
Noninvasive ventilation (NIV) is defined as respiratory support without establishing a tracheal airway. It may be achieved by either negative pressure ventilation or positive pressure ventilation via a mask or similar device.
This requires the application of subatmospheric pressure to the trunk. It was first reported in 1864 using a subject seated in a rigid box connected to a manually operated piston, and following the development of automatic machines it was widely used during polio epidemics in the 1950s. Enthusiasm for the technique has fluctuated since, but there continues to be interest in negative pressure ventilation for a small group of patients.
Animal studies comparing negative and positive pressure ventilation show that lung perfusion is the same with both modes, but that ventilation is more evenly distributed and oxygenation better with negative pressure ventilation. Negative pressure ventilation continues to have a place in the management of respiratory failure resulting from neuromuscular disorders, central apnoeas or in paediatric intensive care.
A cabinet ventilator, often referred to as an iron lung, requires the whole body except the head to be encased in a cabinet with an airtight seal around the neck. An intermittent negative pressure is then applied in the tank, causing inspiration, with passive expiration as normal. A superimposed continuous negative pressure may also be applied, which provides the negative pressure equivalent of positive end-expiratory pressure (PEEP). In terms of the airway-to-ambient pressure gradient, cabinet ventilators are identical in principle to positive pressure ventilation, with similar effects on cardiovascular and respiratory physiology. Collapse of the extrathoracic upper airway during inspiration may occur, particularly during sleep. Vomiting or regurgitation of gastric contents exposes the patient to the danger of aspiration during the inspiratory phase.
Cuirass and jacket ventilators are a simplified form of cabinet ventilators in which the application of subatmospheric pressure is confined to the trunk or anterior abdominal wall. Function depends on a good airtight seal. They are less efficient than cabinet ventilators and suffer from the same disadvantages. However, they are much more convenient to use and may be useful to supplement inadequate spontaneous breathing.
Positive pressure ventilation may be delivered using soft masks that fit over the mouth and nose or the nose only or using a clear plastic helmet that fits over the entire head (sealed around the neck). Most ventilator systems used are pressure generators, and so are ‘leak-tolerant’; that is, flow automatically increases to compensate for a pressure drop because of gas leakage, but there is variation in how well this is achieved between different ventilators and ventilation modes. With nasal ventilation, positive pressure in the nasopharynx normally displaces the soft palate anteriorly against the tongue, thus preventing escape of gas through the mouth.
Complications of NIV include nasal skin damage or ulceration, gastric distension, claustrophobia and discomfort. Helmet systems avoid some of these problems, but have a volume of around 10 L, which inevitably causes some rebreathing, making hypercapnia a common complication. The high volume in the helmet also results in a time delay when changing the pressure in the helmet to support ventilation or when sensing a spontaneous breath with pressure changes (see later).
Techniques of ventilation are similar to invasive artificial ventilation. Ventilator modes that use patient triggering are better tolerated than controlled ventilation, particularly in awake patients. Pressure-controlled ventilation (PCV) or pressure support ventilation (PSV, see later) is commonly used along with CPAP. In bilevel positive airway pressure the ventilator pressure steps between two preset values for inspiration and expiration, and, except for the terminology used to describe the pressures, is the same as PSV with CPAP.
Ventilation may be provided continually during acute respiratory problems, or only at night for long-term respiratory disease. The use of nasal CPAP for treating sleep-disordered breathing has been described on page 197. In this case benefit occurs simply by displacing the soft palate away from the posterior pharyngeal wall. Benefit in other respiratory diseases is more difficult to explain, but in the COVID-19 pandemic of 2020 (page 352), CPAP (with pragmatic arterial blood gas targets for gas exchange) was reported to be particularly effective at preventing patients progressing to artificial ventilation. Possible mechanisms for a benefit of NIV include:
Resting fatigued respiratory muscles
Delivery of a higher inspired oxygen concentration by the use of a tight-fitting facemask
Augmentation of minute ventilation to reduce hypercapnia
Prevention or reexpansion of areas of atelectasis, as seen when using PEEP (see later)
Reduction of cardiac preload in patients with heart failure (page 391)
NIV is now advocated for the treatment of acute respiratory failure from numerous causes, , although outcome evidence supporting its use is variable as follows:
COPD exacerbations ( page 331 ) . NIV is now recommended for treatment of COPD exacerbation leading to respiratory acidosis, but not for other, less severe, exacerbations.
Cardiogenic pulmonary oedema . This may be successfully treated with NIV, reducing the need for tracheal intubation and improving mortality. The mechanism of this beneficial effect is explained on page 344.
Acute lung injury (ALI) . NIV instituted early in the course of ALI ( Chapter 31 ) may reduce the need for tracheal intubation, improve gas exchange and lead to improved long-term outcomes in survivors, although data on mortality remains inconclusive.
Weaning from invasive ventilation ( page 385 ) . NIV may be used to gradually wean patients from invasive ventilation, a strategy that is particularly useful in patients with COPD or obesity.
During intermittent positive pressure ventilation (IPPV), the mouth (or airway) pressure is intermittently raised above ambient pressure. The inspired gas then flows into the lungs in accord with the resistance and compliance of the respiratory system. If inspiration is slow, the distribution is governed mainly by regional compliance. If inspiration is fast, there is preferential ventilation of parts of the lungs with short time constants (see Fig. 2.6 ). Different temporal patterns of pressure may be applied, as discussed next.
During IPPV, expiration results from allowing mouth pressure to fall to ambient. Expiration is then passive and differs from expiration during spontaneous breathing in which diaphragm muscle tone is gradually reduced (page 43). Expiration may be impeded by the application of PEEP. In the past, expiration was sometimes accelerated by the application of a subatmospheric pressure, termed negative end-expiratory pressure , although this technique is no longer used. Expiration to ambient pressure is termed zero end-expiratory pressure (ZEEP).
If the inflating pressure is maintained for several seconds, the resulting tidal volume will be indicated by the following relationship:
Thus for example, a sustained inflation pressure of 10 cmH 2 O with a static compliance of 0.5 L.kPa –1 (50 mL.cmH 2 O –1 ) would result in a lung volume 500 mL above functional residual capacity (FRC).
Equilibration according to the previous equation usually takes several seconds. When the airway pressure is raised during inspiration, it is opposed by the two forms of impedance: the elastic resistance of lungs and chest wall ( Chapter 2 ) and resistance to air flow ( Chapter 3 ). At any instant, the inflation pressure equals the sum of the pressures required to overcome these two forms of impedance. The pressure required to overcome elastic resistance equals the lung volume above FRC divided by the total (dynamic) compliance, whereas the pressure required to overcome air flow resistance equals the air flow resistance multiplied by the instantaneous flow rate.
The effect of applying a constant pressure (PCV) is shown in Figure 32.1 . The two components of the inflation pressure vary during the course of inspiration, while their sum remains constant. The component overcoming air flow resistance is maximal at first and declines exponentially with air flow as inflation proceeds. The component overcoming elastic resistance increases with the lung volume. With normal respiratory mechanics in the unconscious patient, the change in lung volume should be 95% complete in about 1.5 seconds, as in Figure 32.1 .
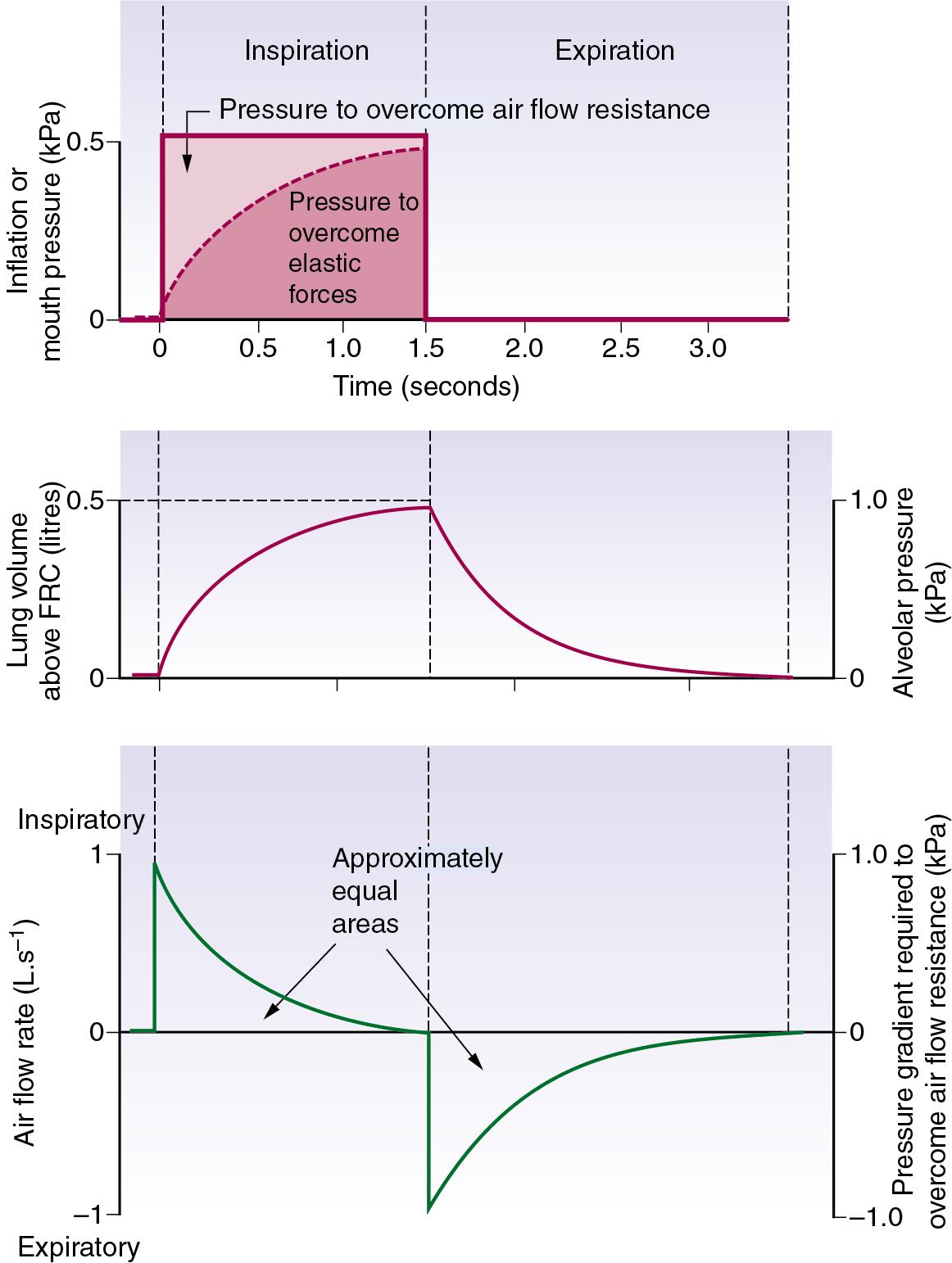
The approach of the lung volume to its equilibrium value is according to an exponential function of the wash-in type (see Appendix E ). The time constant, which is the time required for inflation to 63% of the equilibrium value, equals the product of resistance and compliance. Normal values for an unconscious patient are as follows:
or
The time constant is the time that would be required to reach equilibrium if the initial inspiratory flow rate were maintained. It is sometimes more convenient to use the half-time, which is 0.69 times the time constant. The inflation curve is shown in full with further mathematical detail in Appendix E .
It is normal practice for the inspiratory phase to be terminated after 1 or 2 seconds, at which time the lung volume will still be increasing. Inflation pressure is not then the sole arbiter of tidal volume but must be considered in relation to the duration of the inspiratory phase.
If expiration is passive and mouth pressure remains at ambient, the driving force is the elevation of alveolar pressure above ambient, which is caused by elastic recoil of lungs and chest wall. This pressure is dissipated in overcoming air flow resistance during expiration. In Figure 32.1 , during expiration the alveolar pressure (proportional to the lung volume above FRC) is directly proportional to expiratory flow rate, and all three quantities decline according to a wash-out exponential function with a time constant which is again equal to the product of compliance and resistance.
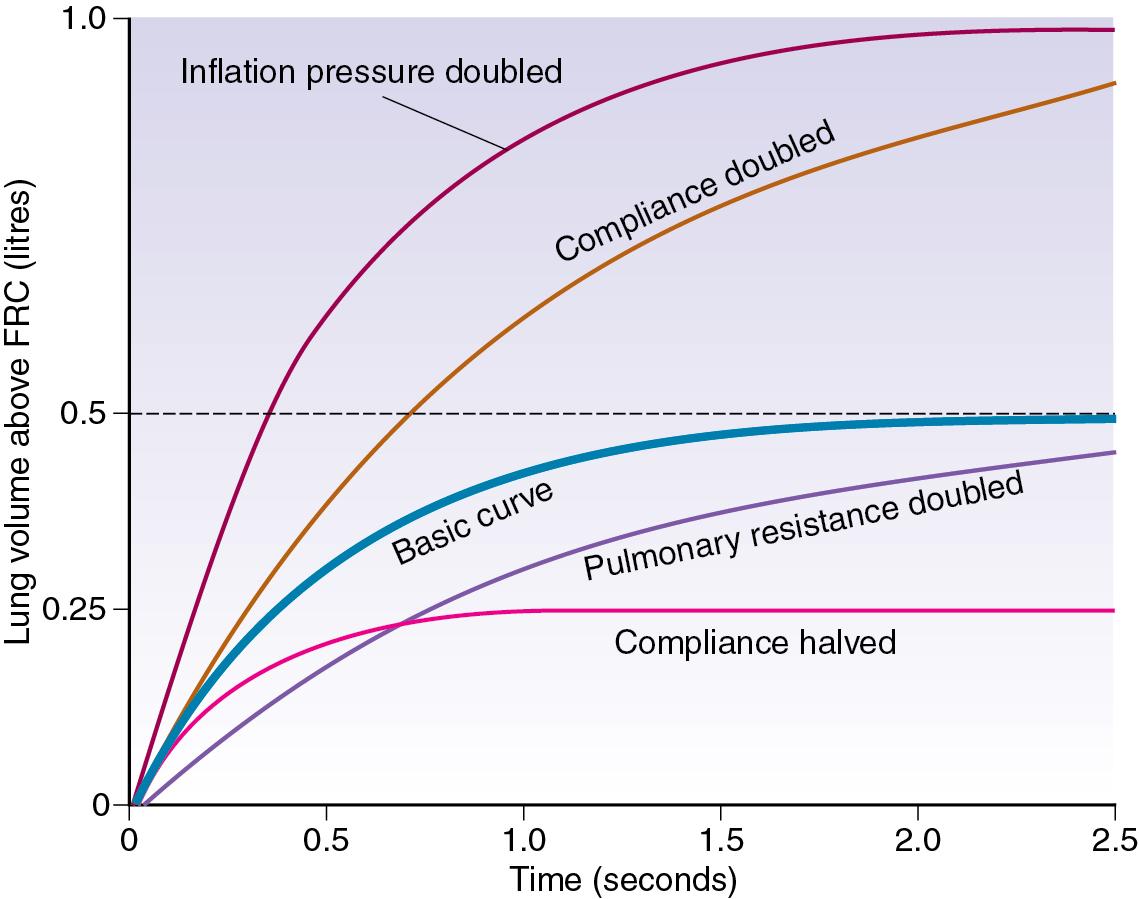
The blue line in Figure 32.2 shows the inflation curve for the normal parameters of an unconscious paralysed patient, as listed in Table 32.1 . These are the same values that were considered earlier. The basic curve is a single exponential approaching a lung volume 0.5 L above FRC with a time constant of 0.5 seconds. Changes in inflation pressure do not alter the time constant of inflation, but directly influence the amount of air introduced into the lungs in a given number of time constants. In Figure 32.2 , each point on the red curve labelled ‘inflation pressure doubled’ is twice the height of the corresponding point on the basic curve for the same time.
| Basic Curve | Pulmonary Resistance Doubled | Inflation Pressure Doubled | Compliance Doubled | Compliance Halved | |
|---|---|---|---|---|---|
| Inflation pressure | |||||
| (kPa) | 1 | 1 | 2 | 1 | 1 |
| (cmH 2 O) | 10 | 10 | 20 | 10 | 10 |
| Compliance | |||||
| (L.kPa –1 ) | 0.5 | 0.5 | 0.5 | 1 | 0.25 |
| (mL.cmH 2 O –1 ) | 50 | 50 | 50 | 100 | 25 |
| Final tidal volume (L) | 0.5 | 0.5 | 1 | 1 | 0.25 |
| Pulmonary resistance | |||||
| (kPa.L –1 .s) | 1 | 2 | 1 | 1 | 1 |
| (cmH 2 O.L –1 .s) | 10 | 20 | 10 | 10 | 10 |
| Time constant | |||||
| (s) | 0.5 | 1 | 0.5 | 1 | 0.25 |
If the compliance is doubled, the equilibrium tidal volume is also doubled. However, the time constant (product of compliance and resistance) is also doubled; therefore, the equilibrium volume is approached more slowly ( Fig. 32.2 ). Conversely, if the compliance is halved, the equilibrium tidal volume is also halved along with the time constant.
Changes in resistance have a direct effect on the time constant of inflation, but do not affect the equilibrium tidal volume. Thus the effect of an increased resistance on tidal volume is through the reduction in inspiratory flow rate. Within limits, this can be counteracted by prolonging inspiration or by increasing the inflation pressure and the degree of overpressure (explained later). The effects, shown in Figure 32.2 , apply not only to the whole lung but also to regions that may have different compliances, resistances and time constants (page 90).
Increasing the inflation pressure has a major effect on the time required to achieve a particular lung volume above FRC. In Figure 32.3 , the lung characteristics are the same as for the ‘basic curve’ in Figure 32.2 . If the required tidal volume is 475 mL, this is achieved in 1.5 seconds with an inflation pressure of 10 cmH 2 O. However, the same lung volume is achieved in only 0.3 seconds by doubling the inflation pressure. The application of a pressure that, if sustained, would give a tidal volume higher than that which is intended is known as overpressure; it is used extensively to increase the inspiratory flow rate to shorten the inspiratory phase. The use of a subatmospheric pressure to increase the rate of passive expiration is similar in principle but is complicated by air trapping ( Fig. 32.3 , B ).

It is helpful to assume that the patterns of air flow previously described are exponential in character, as this greatly assists our understanding of the situation. However, there are many reasons why air flow should not be strictly exponential in character. Air flow is normally partly turbulent (see Chapter 3 ); therefore, resistance cannot be considered as a constant. Furthermore, as expiration proceeds, the calibre of the air passages decreases, and there is also a transition to more laminar flow as the instantaneous flow rate decreases. Approximation to a single exponential function is nevertheless good enough for many practical purposes.
Constant pressure or square wave inflation was previously considered because it is the easiest for mathematical analysis. There are, however, a variety of pressure profiles that may be applied for IPPV. There is no convincing evidence of the superiority of one over the other, except that distribution of inspired gas is improved if there is a prolongation of the period during which the applied pressure is maximal. This permits better ventilation of the ‘slow’ alveoli.
Constant flow rate ventilators (volume-controlled ventilation [VCV]) are extensively used, and Figure 32.4 shows pressure, volume and flow changes in a manner analogous to Figure 32.1 .
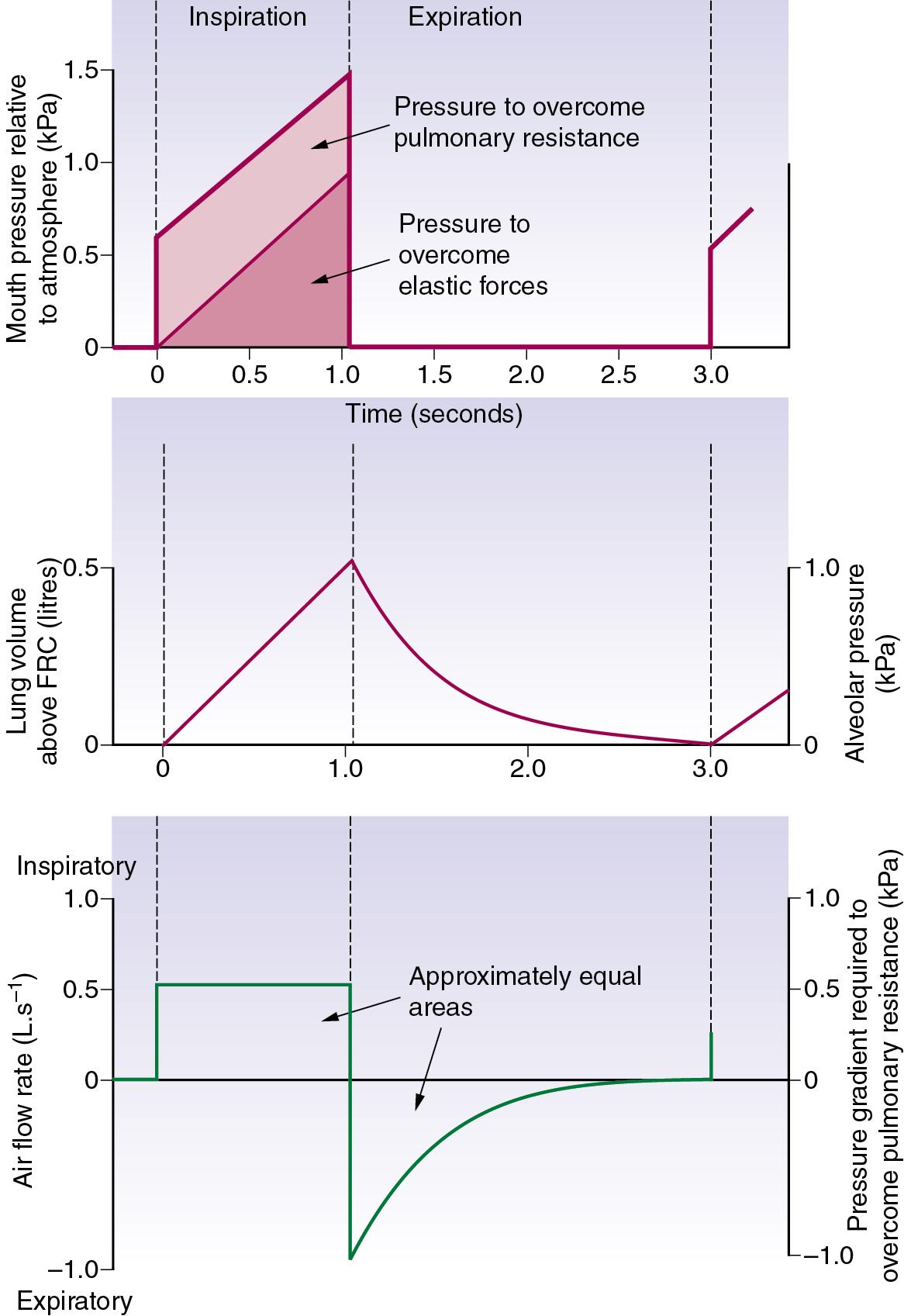
Three methods are in general use.
Time cycling terminates inspiration after a preset time, irrespective of whether inspiration is achieved by constant pressure or constant flow generation. With constant flow generators, inspiratory time has a direct effect on the tidal volume. With constant pressure generators the relationship is more complex, as described earlier (see Fig. 32.3 ).
Volume cycling terminates inspiration when a preset volume has been delivered. In the absence of a leak this should guarantee the tidal volume even if the compliance or resistance of the respiratory system changes within limits.
Pressure cycling terminates inspiration when a particular airway pressure is achieved. This in no way guarantees the tidal volume. Increased airway resistance, for example, would limit inspiratory flow rate and cause a more rapid increase in mouth pressure, thus terminating the inspiratory phase. Pressure-cycled ventilators are almost invariably flow generators.
Limitations on inspiratory duration. Whatever the means of cycling, it is possible to add a limitation on inspiratory duration, usually as a safety precaution. For example, a pressure limitation can be added to a time-cycled or a volume-cycled ventilator. This can either function as a pressure relief valve or it can terminate the inspiratory phase.
For a given minute volume of ventilation, it is possible to vary within wide limits the duration of inspiration and expiration and the ratio between the two. A common pattern is about 1 second for inspiration, followed by 2 to 4 seconds for expiration (inspiratory to expiratory [I:E] ratio 1:2–1:4), giving respiratory frequencies in the range of 12 to 20 breaths per minute. The problem is whether changes from this pattern confer any appreciable benefit in terms of gas exchange. Reduction of the inspiratory time to less than 1 second may cause an increase in dead space, but there is no evidence that the duration of inspiration (in the range of 0.5–3 seconds) has any appreciable effect on the alveolar/arterial P o 2 gradient. Thus the accepted view seems to be that 1 second is a reasonable minimal time for inspiration.
Inverse I:E ratio ventilation increases the mean lung volume and may be expected to achieve some of the advantages of PEEP as considered later. It may be achieved either by slowing the inspiratory flow rate (shallow ramp) or by holding the lung volume at the end of inspiration (inspiratory pause); the latter seems to be more logical. I:E ratios as high as 4:1 have been used, but 2:1 is generally preferable. The degree of inverse I:E ratio used is limited by the cardiovascular disturbances seen with the technique (see later discussion) and the time available for expiration. If the latter is unduly curtailed, FRC will be increased, generating so-called ‘intrinsic-PEEP’ (see later).
Gas redistribution during an inspiratory hold reduces the dead space (page 97), resulting in a lower P co 2 for the same minute volume. This permits the use of a lower peak inflation pressure.
The previous section classifies ventilators according to the method of gas flow generation (for example, constant flow or constant pressure generators) based on the mechanism by which the ventilator worked. Most ventilators in clinical use are now electronically controlled. These allow accurate control of gas pressure and flow throughout the ventilator circuit and can normally perform as either flow or pressure generators with various inspiratory flow patterns. In addition, they have given rise to a whole host of previously impossible ventilatory techniques, a majority of which are dependent on the ventilator responding appropriately to the patient’s own respiratory efforts.
For many years there have been ventilators in which the inspiratory phase could be triggered with a spontaneous breath, and mechanical ventilators could be modified to facilitate a mandatory minute volume (MMV) of ventilation, as described next. Electronic ventilators continuously monitor tidal volume, whether generated by the patient (spontaneous breath) or artificially (ventilator breath). With this information available it is a simple task to achieve, by electronic means, a predetermined minute volume, number of breaths, and so on, by introducing extra ventilator breaths when necessary. The challenge for ventilator design in recent years has been the speed and sensitivity with which ventilators can sense, and respond to, the patient’s respiratory efforts to synchronize ventilator and spontaneous breaths. Without this synchronization, a patient with any reasonable spontaneous respiratory effort begins to ‘fight’ against the ventilator. Some degree of asynchrony occurs in all ventilated patients, and this leads to discomfort, poor gas exchange, cardiovascular disturbance and worse clinical outcomes.
There are three ways by which a ventilator may detect the onset of a spontaneous breath as follows.
At the onset of a respiratory effort, the patient will generate a reduction in pressure within the circuit, which may be detected in the ventilator. This pressure wave travels through the circuit at approximately the speed of sound, reaching the ventilator within 12 ms, following which the pressure sensor must respond, and flow into the circuit be increased to facilitate inspiration. Overall, these events take approximately 100 ms to occur, which is undetectable by the patient. The pressure drop required to trigger inspiration is now always measured relative to circuit (not atmospheric) pressure, to allow the use of CPAP/PEEP during ventilation. The time taken to trigger the ventilator increases with decreased sensitivity settings, that is, when a greater pressure drop is required for triggering.
Detection of inspiratory flow may trigger a ventilator breath or some type of respiratory assist (see later). Most current intensive care ventilators provide a continuous base flow around the ventilator circuit of 2 to 20 L.min −1 . Any difference between ventilator inflow and outflow represents the patient’s respiration. Flow triggering occurs in approximately 80 ms, irrespective of the sensitivity setting. A high base flow provides adequate inspiratory flow for the patient at the start of inspiration, and the flow rate is increased when the ventilator is triggered. Flow sensing can also detect the end of inspiration and is used in PSV (see later).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here