Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Hormonal changes of pregnancy stimulate breathing, causing an increase in tidal volume and hypocapnia.
In late pregnancy the enlarged uterus reduces lung volume, particularly in the supine position.
Human lung development is incomplete at birth, with new alveoli continuing to form until around 3 years of age.
Compared with adults, the respiratory system of a neonate has a very low compliance and a high resistance.
In children, most measures of lung function are the same as adults, provided the values are related to lung volume or height.
Several physiological changes occur during pregnancy that affect respiratory function. Fluid retention resulting from increasing oestrogen levels causes oedema throughout the airway mucosa and increases blood volume, substantially increasing oxygen delivery. Progesterone levels rise six-fold through pregnancy and have significant effects on the control of respiration, and therefore arterial blood gases. Finally, in the last trimester of pregnancy, the enlarging uterus has a direct impact on respiratory mechanics. A summary of the changes for common respiratory measurements is shown in Table 12.1 .
Lung volumes. During the last third of pregnancy the diaphragm becomes displaced cephalad by the expansion of the uterus into the abdomen. This reduces both the residual volume (by about 20%) and expiratory reserve volume, such that functional residual capacity (FRC) is greatly reduced ( Table 12.1 ). This is particularly true in the supine position, and effectively removes one of the largest stores of oxygen available to the body, making pregnant women very susceptible to hypoxia during anaesthesia or if they have respiratory disease. Vital capacity, forced expiratory volume in one second and maximal breathing capacity are normally unchanged during pregnancy.
Oxygen consumption. Oxygen consumption increases throughout pregnancy, peaking at between 15% and 30% above normal at full term. The increase is mainly attributable to the demands of the fetus, uterus and placenta, such that when oxygen consumption is expressed per kilogram of body weight there is little change.
Ventilation. Respiratory rate remains unchanged, whereas tidal volume, and therefore the minute volume of ventilation, increases by up to 40% above normal at full term. The increase in ventilation is beyond the requirements of the enhanced oxygen uptake or carbon dioxide production, so alveolar and arterial P co 2 are reduced to about 4 kPa (30 mmHg). There is also an increase in alveolar and arterial P o 2 of about 1 kPa (7.5 mmHg). Both these changes will facilitate blood gas exchange across the placenta.
| Pregnant | ||||
| Variable | Nonpregnant | 1st Trimester | 2nd Trimester | 3rd Trimester |
| Tidal volume (L) | 0.52 | 0.60 | 0.65 | 0.72 |
| Respiratory rate (breaths per minute) | 18 | 18 | 18 | 18 |
| Minute volume (L.min −1 ) | 9.3 | 11.0 | 11.8 | 13.1 |
| Residual volume (L) | 1.37 | 1.27 | 1.26 | 1.01 |
| Functional residual capacity (L) | 2.69 | 2.52 | 2.48 | 1.95 |
| Vital capacity (L) | 3.50 | 3.45 | 3.58 | 3.0 |
| Oxygen consumption (mL.min −1 ) | 194 | 211 | 242 | 258 |
| Arterial P o 2 (kPa) | 12.6 | 14.2 | 13.7 | 13.6 |
| (mmHg) | 95 | 106 | 103 | 102 |
| Arterial P co 2 (kPa) | 4.70 | 3.92 | 3.93 | 4.05 |
| (mmHg) | 35 | 29 | 29 | 31 |
| Carbon dioxide response slope (L.min −1 .kPa −1 ) | 11.6 | 15.0 | 17.3 | 19.8 |
| Oxygen saturation response slope (L.min −1 .% −1 ) | 0.64 | 1.04 | 1.13 | 1.33 |
Hyperventilation is attributable to progesterone, and the mechanism is assumed to be a sensitization of the central chemoreceptors. Pregnancy gives rise to a threefold increase in the slope of a P co 2 /ventilation response curve. The hypoxic ventilatory response is increased twofold, with most of the change occurring before the midpoint of gestation, at which time oxygen consumption has hardly begun to increase.
Dyspnoea occurs in more than half of pregnant women, often beginning early in pregnancy, before the mass effect of the uterus becomes apparent. Dyspnoeic pregnant women, compared with nondyspnoeic controls, show a greater degree of hyperventilation in spite of having similar plasma progesterone levels. Dyspnoea early in pregnancy therefore seems to arise from a greater sensitivity of the chemoreceptors to the increase in progesterone levels. In the third trimester, when dyspnoea on mild exercise is almost universal, the extra effort required by the respiratory muscles to increase tidal volume is believed to be responsible for breathlessness, rather than an altered perception of respiratory discomfort.
The lungs develop in four stages, under the control of a host of transcriptional factors: ,
Pseudoglandular stage (5–17 weeks’ gestation). A ventral outgrowth from the foregut first appears about 24 days after fertilization, and at around week 5 of gestation this begins to form the basic airway and vascular architecture. The branching patterns of the adult airways and vasculature are believed to develop by a common process of branching morphogenesis, ensuring the two components remain closely related in the lung tissue. Dividing epithelial cells lengthen the airways, and their ability to do this is influenced by physical factors relating to the lung liquid (LL) and fetal breathing described next.
Canalicular stage (16–26 weeks’ gestation). The primitive pulmonary capillaries now become more closely associated with the airway epithelium, and the connective tissue architecture of the lung is formed. Fibroblasts and other cells involved in morphogenesis of the lung undergo apoptosis, reducing the wall thickness of the embryonic lung structures.
Saccular stage (24 weeks’ gestation to term). Distal airways now develop primitive alveoli in their walls to become respiratory bronchi (see Fig. 1.6 ). Saccules form at the termination of airways, these being primitive pulmonary acini.
Alveolar stage. Saccules on embryonic bronchioles now expand, and septation occurs to form the groups of alveoli seen in adult pulmonary acini. This phase of development begins at 36 weeks’ gestation, and in humans is believed to be mostly complete around 2 years of age, although there is some evidence the process may continue throughout childhood, particularly in infants born prematurely. In humans at full term all major elements of the lungs are therefore fully formed, but the number of alveoli present is only about 15% of that in the adult lung. This postnatal maturation of lung structure is only seen in altricial mammals (humans, mice and rabbits) that have the luxury of being able to remain ‘helpless’ after birth. Precocial species such as range animals are born with a structurally mature lung, ready for immediate activity. Once alveolarization is complete, further lung growth is dimensional, that is, simple enlargement of existing structures.
The lungs begin to contain surfactant and are first capable of function by approximately 24 to 28 weeks, this being a major contributor to the viability of premature infants, although the factors influencing surfactant development, particularly of the surfactant proteins, are poorly understood.
Fetal lungs contain LL which is secreted by the pulmonary epithelial cells and flows out through the developing airway into the amniotic fluid or gastrointestinal tract, flushing debris from the airways as it does so. A more important function of LL seems to be to prevent the developing lung tissues from collapsing. It is thought that LL maintains the lung at a slight positive pressure relative to the amniotic fluid, and that this expansion is responsible for stimulating cell division and lung growth, particularly with respect to airway branching. The respiratory tract in late pregnancy contains about 40 mL of LL, but its turnover is rapid, believed to be of the order of 500 mL per day. Its volume corresponds approximately with the FRC after breathing is established.
Fetal breathing movements also contribute to lung development. In humans they begin in the middle trimester of pregnancy, and are present for over 20 minutes per hour in the last trimester, normally during periods of general fetal activity. During episodes of breathing, the frequency is about 45 breaths per minute, and the diaphragm seems to be the main muscle concerned, producing an estimated fluid shift of about 2 mL at each ‘breath’.
Maintenance of a positive pressure in the developing lung requires the upper airway to offer some resistance to the outflow of LL. During apnoea, elastic recoil of the lung tissue and continuous production of LL are both opposed by intrinsic laryngeal resistance and a collapsed pharynx. Fetal inspiratory activity, as in the adult, includes dilation of the upper airway. With quiet breathing this would allow increased efflux of LL from the airway, but simultaneous diaphragmatic contraction opposes this. During vigorous breathing movements with the mouth open, pharyngeal fluid may be ‘sucked’ into the airway, thus contributing to the expansion of the lungs. Thus fetal breathing movements are believed to contribute to maintaining lung expansion, and their abolition is known to impair lung development.
As the lungs develop in utero, they face a variety of environmental challenges associated with the pregnancy that can affect their development and influence the individual’s lung function in both childhood and adult life. Adverse effects on lung function arising during pregnancy include a greater likelihood of developing childhood wheeze (which may or may not be asthma), developing asthma or having lower lung volumes as an adult. The more well-studied factors contributing to these lung problems include:
Low birth weight . This is associated with lower lung volumes as both a child and adult, with the greatest effect seen when aged 21 years, and results in a greater likelihood of developing asthma and requiring hospitalization for respiratory disease as an adult. The cause of these effects remains uncertain, but it most likely results from poor fetal lung growth in the first trimester, although early postnatal growth also influences future lung health.
Prematurity . Birth at less than 37 weeks’ gestation leads to lower lung volumes in childhood, with values lower still in neonates who develop and survive bronchopulmonary dysplasia (page 180). Pulmonary gas transfer remains lower than control subjects at 21 years of age, although this does not adversely affect exercise capacity.
Maternal tobacco smoking . This leads to lower birth weight and earlier birth, meaning these babies will therefore have poorer lung function. Babies whose mothers smoked during pregnancy also have a higher incidence of childhood wheeze and asthma, with smoking during the first trimester having the greatest effect; of course postnatal passive smoking also plays a role (page 239). Nicotine is believed to play a major role in the effects of smoking on in utero lung development, leading to concern that use of e-cigarettes (page 239) may be similarly harmful to the fetus. In utero smoke exposure also causes epigenetic changes in the fetus (resulting from changes to DNA by its methylation) that mean a susceptibility to respiratory disease may be passed down to subsequent generations.
Maternal stress during pregnancy . Stress, particularly in overweight mothers, leads to children being more likely to develop wheeze at 2 to 3 years old. Raised maternal cortisol levels may directly influence early lung development, but maternal stress also has much broader effects on fetal well-being relating to placental function and immune system development.
Indoor air pollution (page 242). Prenatal exposure to indoor carbon monoxide is associated with impaired lung function for the child in the first year of life.
The fetal circulation differs radically from the postnatal circulation ( Fig. 12.1 ). Blood from the right heart is deflected away from the lungs, partly through the foramen ovale and partly through the ductus arteriosus. Less than 10% of the output of the right ventricle reaches the lungs, the remainder passing to the systemic circulation and the placenta. Right atrial pressure exceeds left atrial pressure, and this maintains the patency of the foramen ovale. Finally, because the vascular resistance of the pulmonary circulation exceeds that of the systemic circulation before birth, pressure in the right ventricle exceeds that in the left ventricle, and these factors control the direction of flow through the ductus arteriosus.
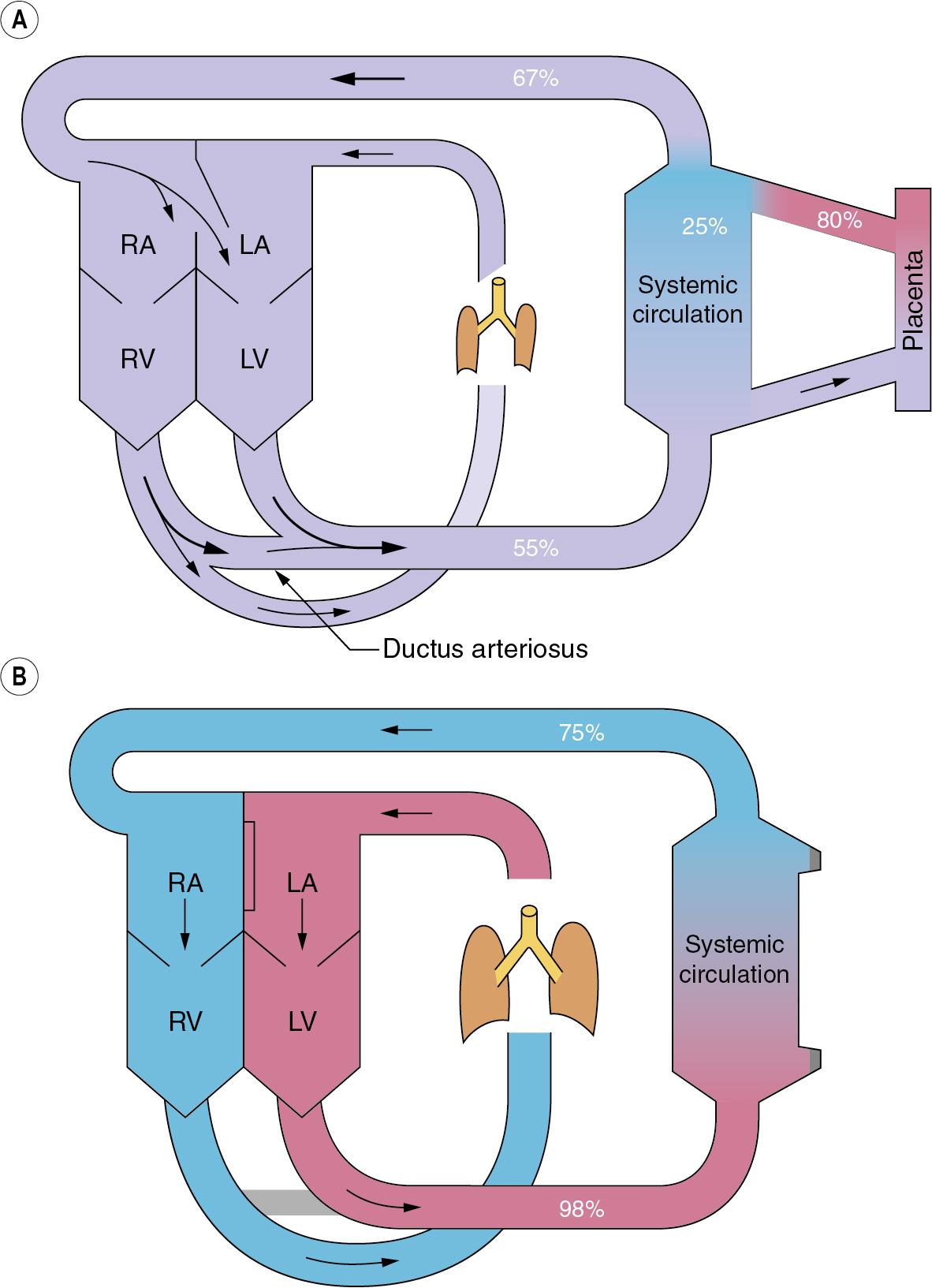
The umbilical veins drain via the ductus venosus into the inferior vena cava, which contains better-oxygenated blood than the superior vena cava. The anatomy of the atria and the foramen ovale is such that the better-oxygenated blood from the inferior vena cava passes preferentially into the left atrium, and then to the left ventricle, and so to the brain. (This is not shown in Fig. 12.1 ). Overall gas tensions in the fetus are of the order of 6.4 kPa (48 mmHg) for P co 2 and 4 kPa (30 mmHg) for P o 2 . The fact that the fetus remains apnoeic for much of the time in utero with these blood-gas levels is probably in part attributable to central hypoxic ventilatory depression (page 53).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here