Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Nonmalignant vascular anomalies can be functionally divided into two groups: vascular tumors and vascular malformations. Unfortunately, this distinction is not universally appreciated, due to “terminologic imprecision.” Despite efforts by vascular anomalies specialists to publicize the accurate lexicon, the term hemangioma (a vascular tumor) is often incorrectly applied to describe vascular malformations.
The original classification proposed by Mulliken and Glowaki has since been updated to include syndromic vascular anomalies, newly recognized diagnoses, and genetic mutations by The International Society for the Study of Vascular Anomalies (ISSVA) ( http://www.issva.org/classification ), an international group of vascular anomalies specialists ( Table 64.1 and Box 64.1 ). This group will regularly update the classification as new information emerges.
| Vascular tumors | Vascular malformations | |||
|---|---|---|---|---|
| Benign | Locally Aggressive | Malignant | Simple | Combined |
| Infantile hemangioma | Kaposiform hemangioendothelioma | Angiosarcoma | Capillary malformation (CM) | CVM, CLM |
| Congenital hemangioma | Retiform hemangioendothelioma | Epithelioid hemangioendothelioma | Lymphatic malformation (LM) | LVM, CLVM |
| Tufted hemangioma | PILA, Dabska tumor | Venous malformation (VM) | CAVM | |
| Spindle-cell hemangioma | Composite hemangioendothelioma | Arteriovenous malformation (AVM) | CLAVM | |
| Epithelioid hemangioma | Kaposi sarcoma | Arteriovenous fistula | ||
| Pyogenic granuloma | ||||
| Vascular Tumors | Vascular Malformations |
|---|---|
| Infantile hemangiomas Congenital hemangioma Rapidly involuting congenital hemangioma (RICH) Non-involuting congenital hemangioma (NICH) Tufted angioma (± Kasabach-Merritt syndrome) Kaposiform hemangioendothelioma (± Kasabach-Merritt syndrome) Spindle cell hemangioendothelioma Other, rare hemangioendotheliomas (epithelioid, composite, retiform, polymorphous, Dabska tumor, lymphangioendotheliomatosis, etc.) Acquired vascular tumors (pyogenic granuloma, targetoid hemangioma, glomeruloid hemangioma, microvenular hemangioma, etc.) |
Capillary malformation (CM) Port wine stain Telangiectasia Angiokeratoma Venous malformation (VM) Common sporadic VM Familial cutaneous and mucosal venous malformation (VMCM) Glomuvenous malformation (GVM) (glomangioma) Blue rubber bleb syndrome Maffucci syndrome Lymphatic malformation (LM) Fast-flow vascular malformations: Arterial malformation (AM) Arteriovenous fistula (AVF) Arteriovenous malformation (AVM) Complex-combined vascular malformations: CVM, CLM, LVM, CLVM, AVM-LM, CM-AVM |
Hemangiomas are considered the most common tumors of childhood. They are benign growths of endothelial cells with a unique natural history, characterized by a rapid growth phase usually beginning in the first weeks of life and continuing until 9 to 12 months of age ( Fig. 64.1 ). Most hemangiomas subsequently undergo a spontaneous, gradual, but extensive involution. It is recommended to have infants with hemangiomas evaluated for early therapy to prevent further growth and expedite an earlier, more complete, involution. This will ideally minimize associated morbidities and abrogate the need for surgical intervention in the future.

The precise pathophysiology of hemangioma development is not fully defined; however, it is generally accepted that a CD133 hemangioma stem cell is targeted in utero to develop into a hemangioma postnatally in response to growth factors and environmental conditions. Initially, GLUT1, LYVE-1, and Lewis Y-positive endothelial cells are the predominant phenotype. During involution, apoptosis-related changes occur, dominated by adipocytes and fibrous tissue ( Fig. 64.2 ). One retrospective observational study hypothesized that the anatomic distribution of some extremity hemangiomas correlate with embryonic arterial variants which may cause transient focal intrauterine hypoxia that stimulates hemangioma stem cell proliferation ( Fig. 64.3 ). Less commonly, GLUT-1-positive segmental reticular telangiectatic hemangiomas (usually on the lower and distal extremities) can be associated with minimal cutaneous growth (so-called abortive hemangiomas or infantile hemangiomas with minimal or arrested growth [IH-MAG]) ± soft tissue hypertrophy, and are prone to ulceration. Several studies have documented an increased incidence of parenchymal (usually hepatic) hemangiomas when greater than five cutaneous hemangiomas are present. A study of 103 infants with multifocal infantile hemangiomas further categorized the lesions as miliary (small, focal, round, numerous) or nonmiliary (irregular, larger), and found that miliary hemangiomas were more prevalent in premature infants and were more likely to be associated with parenchymal (e.g., hepatic) hemangiomas.


Important exceptions to this growth/regression pattern are the group of rapidly involuting congenital hemangiomas (RICH), which are generally present in full at birth (or even detected prenatally) and spontaneously remit ( Fig. 64.4 ), and noninvoluting congenital hemangiomas (NICH) which do not change size postnatally. Growth curves for these hemangiomas are illustrated in Fig. 64.5 . A subset of patients with RICH may have high-flow lesions with prenatal or postnatal high-flow characteristics and/or transient coagulopathy.
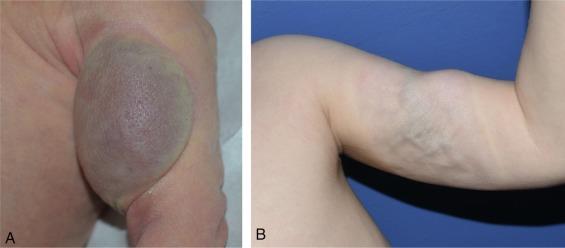

“Congenital nonprogressive hemangiomas” have been shown by North and colleagues to be histologically and immunophenotypically distinct from classical hemangiomas of infancy and are speculated to have a differing pathogenesis. NICH-type lesions were found to have high flow clinically (as assessed by Doppler) and inferred histologically (in that small arteries were seen “shunting” into lobular vessels or abnormal veins). Another subtype of hemangiomas are those with “minimal or arrested growth,” presenting as areas of telangiectasia with peripheral bulkiness. In one series, most of this type of hemangioma were present on the lower extremities.
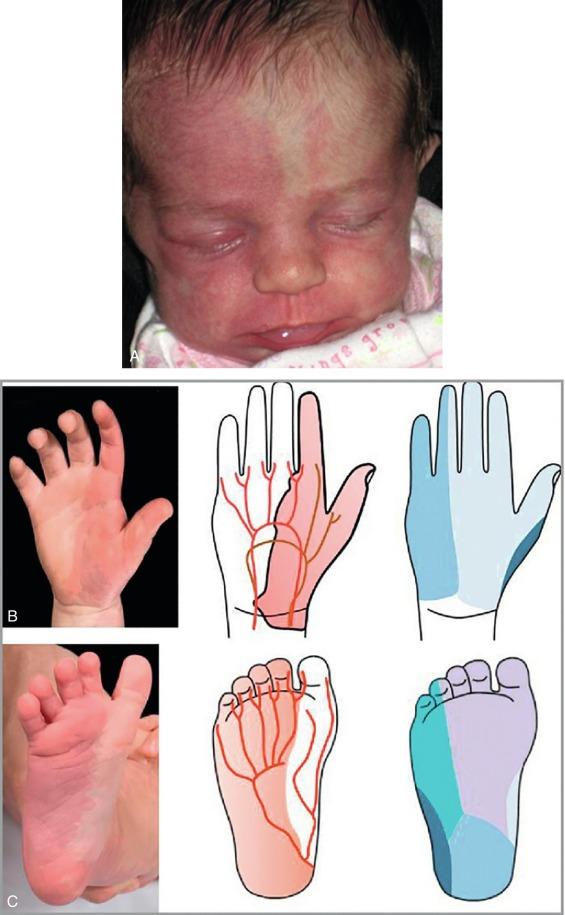
“Typical” hemangiomas are known to be most common in females, premature infants, and in the facial region. Several studies have shown an increased incidence in white non-Hispanic infants, multiple gestation, infants born to older mothers, and in association with placenta previa and/or pre-eclampsia. Hemangiomas can be focal, multifocal, and/or segmental. Segmental hemangiomas can be associated with a higher incidence of PHACE(S), visceral hemangiomas, underlying lumbosacral anomalies (e.g., occult spinal dysraphism including lipomyelomeningocele with tethered cord). The PHACE(S) association is an acronym for: P osterior fossa structural malformations, H emangiomas (segmental), A rterial anomalies, C ardiac defects, E ye abnormalities, (and S ternal and other midline deformities). A patient with a facial segmental hemangioma (> 5 cm diameter) and one or more of the above criteria has PHACES ( Fig. 64.6 ). Updated consensus-derived criteria for this diagnosis have been reported by Garzon et al. In one series, approximately one-third of patients with facial segmental hemangiomas were found to have PHACES, those at higher risk having large hemangiomas involving more than one anatomical segment, and in the frontonasal or frontotemporal distribution. Of those with PHACES, most (90%) had > 1 extracutaneous finding (most commonly central nervous system [CNS] arteriopathy or cardiac anomaly). Similarly, Oza and colleagues observed that patients with large facial segmental cutaneous (Seg1-Seg4) hemangiomas were especially at risk of CNS structural and cerebrovascular anomalies, those with S1 distribution hemangiomas had a higher incidence of ocular anomalies, and those with S3 distribution had airway, ventral, and cardiac anomalies. In this series, all patients with CNS structural anomalies had concomitant CNS arteriopathies. Also identified were supratentorial CNS anomalies (cortical dysgenesis and migration abnormalities). Arteriopathies are most commonly dysplastic vessels with an aberrant course involving the internal cerebral artery and its embryonic branches, ipsilateral to the side of the cutaneous hemangioma. Hypoplasia, agenesis, or absence of normal arteries can also occur and progressive changes can lead to aneurysm formation or moyamoya-type changes, and one group identified RNF213 variants in a PHACE patient who developed moyamoyavasculopathy. In one study of 55 PHACE patients, 62% had a nonvascular intracranial anomaly, many of whom had structural brain malformations, pituitary anomalies, and intracranial hemangiomas. Developmental delays and hearing loss may be associated with PHACE syndrome; thus, patients should be monitored closely for age-appropriate neurocognitive milestones. Headaches are also more common in patients with PHACE syndrome, and should a patient have a hypoplastic or occlusive cerebral arteriopathy, triptans and other medications that can cause CNS vasoconstriction should be avoided.

Some hemangiomas are small, asymptomatic, and located in areas that are not aesthetically sensitive; these may not require therapy. Hemangioma-associated morbidities include upper airway obstruction, ophthalmologic disturbances, ulceration, bleeding, persistent soft tissue deformity, cerebral vasculopathy, and/or high-output congestive heart failure. Additionally, hemangiomas may be the source of significant psychosocial stress, due to comments by others, and parental fears. Tables 64.2 and 64.3 summarize clinical and therapeutic issues relevant to hemangiomas.
| Clinical Finding | Recommended Evaluation |
|---|---|
| Hemangiomatosis—multiple, small, cutaneous hemangiomas | Evaluate for parenchymal hemangiomas, especially hepatic/CNS/gastrointestinal |
| Cutaneous hemangiomas in “beard” distribution | Evaluate for airway hemangioma, especially presenting with stridor |
| Facial segmental hemangioma involving significant area of face (> 5 cm) | Evaluate for PHACES—MRI ± contrast for orbital hemangioma ± posterior fossa malformation MRA brain, neck to thoracic aorta Cardiac, ophthalmologic evaluations supraumbilical raphe, sternal atresia, cleft |
| Paraspinal midline vascular lesion | Ultrasound (if less than 6 months of age) or MRI to evaluate for occult spinal dysraphism ± underlying vascular lesion |
| Thrill or bruit, or both, associated with hemangioma | Cardiac evaluation and echo to rule out diastolic reversal of flow of aorta MRI/Doppler of vascular lesion to evaluate flow characteristics |
| Large hemangioma Hepatic hemangiomas |
Ultrasound with Doppler MRI + contrast CBC, metabolic panel, Thyroid function studies, quantitative α fetoprotein |
| Preferential position (e.g., torticollis) | Consider physical therapy evaluation |
| Delayed milestones | Consider CNS issues, hearing evaluation |
| Clinical Finding | Recommended Treatment |
|---|---|
| Hemangiomas—severe ulceration/maceration | Encourage cleansing regimen twice daily Sterile saline soaks/air drying/non-stick gauze ± Metronidazole gel/Becaplermin gel ± Flashlamp-pulsed dye laser Oral β blocker Analgesics—topical, oral Surgery |
| Hemangioma—ophthalmologic sequelae | Patching therapy as directed by ophthalmologist Oral β blocker |
| Subglottic hemangioma | Laryngoscopy, possible bronchoscopy if not well visualized on laryngoscopy oral β blocker—further intervention depends on response |
| Kaposiform hemangioendothelioma or tufted angioma with Kasabach-Merritt phenomenon | Sirolimus, corticosteroids, vincristine |
| Vascular malformation limb-length discrepancy | Shoe insert vs. epiphysiodesis vs. serial observation Orthopedist must follow |
| Foot size/shoe-size discrepancy | |
| Vascular malformation—pain | Evaluate for phlebolith, infection, obstruction, or deep venous thrombosis Analgesics Anticoagulation if thrombosis May improve with sirolimus ± Nerve block, ± sclerotherapy (if not thrombosis) |
| Chylous ascites | Evaluate cause Drainage/low-fat diet/parenteral nutrition/albumin ± Immmunoglobulin replacement Sirolimus if indicated Other medications |
Propranolol (nonselective β-blocker) has become an accepted and well-studied therapy for the treatment of hemangiomas of infancy. This has revolutionized the therapy for infantile hemangioma warranting treatment, with numerous publications, the majority documenting its efficacy with high strength of evidence. Side effects include cool extremities, sleep disturbances, gastrointestinal symptoms, hypotension, bronchial reactivity, and bradycardia with rare but significant reports of hypoglycemia. Overall, systemic β-blocker therapy is generally well-tolerated, with proper patient selection and parental counseling to prevent undue side effects. Data on neurodevelopment and cognitive function in patients treated with propranolol for hemangiomas of infancy are thus far reassuring. Excellent publications reviewing hemangioma management are provided in an Executive Summary by Darrow et al. and a comprehensive systematic review by Chinnadurai et al. and reviews in Otolaryngology Clinics of North America (February 2018, Volume 51, Issue 1).
Oral propranolol, a nonselective β-blocker, has supplanted the previously used corticosteroid therapy, based upon a serendipitous observation when an infant with a proliferating infantile hemangioma was treated with this medication due to steroid-related cardiac issues. The mechanism of β blockers on hemangiomas of infancy is under investigation. Studies have demonstrated the following effects: (1) inhibition of proliferation (via G0/G1 cell cycle arrest) and chemotactic mobility and differentiation of cultured endothelial cells, (2) vascular endothelial growth factor (VEGF)-induced phosphorylation of VEGF R-2 and other angiogenesis-related pathways. Several groups have shown the renin-angiotensin system is activated in hemangiomas of infancy, with elevated angiotensin converting enzyme (ACE) and angiotensin receptor 2 (ATII) in proliferating hemangioma cells, as well as serum elevations of ACE, ATII, and renin. Decreases in plasma renin and ATII post treatment downregulates this pathway in hemangiomas. Other putative mechanisms involve vasoconstriction resulting from decreased nitric oxide release, cAMP-induced inhibition of VEGF- and bFGF-induced endothelial cell proliferation, or inducing apoptosis via MAPK induction.
Topical β blockers have also been shown to catalyze involution of superficial hemangiomas. Systemic absorption of topical therapy is variable, and dependent upon the size and morphology of the hemangioma, application frequency, and technique. For very premature infants, the amount absorbed may be sufficient to cause symptoms.
Ulcerated hemangiomas may respond to oral propranolol; however, adjunctive local therapies are often required. Recombinant platelet-derived growth factor has been effective for ulcerated hemangiomas; however, a “Black Box” warning limits its use. Various combinations of local and systemic therapies for ulcerated hemangiomas are included in the reviews noted above. Topical or systemic antibiotics may be warranted for superinfected ulcerated hemangiomas, topical and/or analgesics, as well as hemostatic agents’ bleeding.
Hepatic hemangiomas represent a special category. Although many hepatic hemangiomas are asymptomatic, a subset carries a high morbidity and mortality rate. They may be solitary or multiple and may be seen in association with cutaneous hemangiomatosis or be an isolated finding. There are three subtypes—focal, multifocal, and diffuse. The data presented in several recent review articles have demonstrated that focal hepatic hemangiomas are RICH-type hemangiomas, present in utero, and gradually involute without therapy. If symptomatic, highly vascular lesions may require intervention. Multifocal and diffuse hemangiomas are not detected in utero, and grow postnatally, in alignment with the growth curve of hemangiomas of infancy. Multifocal hepatic hemangiomas may transition to diffuse hepatic hemangiomas based on these studies. Diffuse hepatic hemangiomas are uncommon, may be associated with profound consumptive hypothyroidism due to elaboration of type 3-iodothyronine deiodinase and congestive heart failure, and have a high mortality rate. Approximately 50% of patients with diffuse hemangiomas did not have cutaneous hemangiomas. Prompt diagnosis and therapy with systemic β blockers may be very effective.
The indications for and timing of surgery for hemangiomas remain controversial. Some surgeons prefer to defer surgery until the hemangioma has undergone substantial involution, with the rationale that the surgery will be less complex and aesthetically more favorable. Other surgeons advocate early intervention, to possibly prevent medical complications, or to avert the psychological stresses on the patient and/or family. In any case, a well-planned strategy with medical, laser, and surgical management decisions discussed among multidisciplinary physicians can provide excellent results. As noted above, as more effective medical therapies have been discovered, the role of surgery for hemangiomas has diminished. Surgical techniques are not discussed in this chapter.
Trapping of platelets and other blood elements (Kasabach-Merritt phenomenon) has been known to occur in association with a subset of vascular anomalies since it was first described in 1940. This is an extremely important diagnosis, as early detection and rapid evaluation and treatment (if clinically symptomatic) is essential. Kasabach-Merritt phenomenon is not associated with common hemangiomas of infancy but with Kaposiform hemangioendotheliomas (KHE) or tufted angiomas. On examination, the lesion is often edematous, boggy, and ecchymotic ( Fig. 64.7 ). Anatomic predilection is for the chest wall and shoulder, groin extending down the leg, retroperitoneum, or face. The gender distribution tends to be equal. Hematologic features of Kasabach-Merritt phenomenon include thrombocytopenia, hypofibrinogenemia, elevated fibrin degradation products, and elevated D-dimer. Radiologic hallmarks of KHE are cutaneous thickening, diffuse enhancement with ill-defined margins, small feeding/draining vessels, stranding, and hemosiderin deposits. The histologic features of KHE are spindled endothelial cells resembling Kaposi sarcoma (KS, but not associated with HIV infection), abnormal lymphatic-like vessels, microthrombi, hemosiderin, and decreased mast cells and pericytes (which are often seen in hemangiomas). There may be residual tumor after resolution of hematologic abnormalities, and radiologic studies often demonstrate persistent vascular tumors. Residua of KHE-associated tumors may be “dormant” vascular tumors, rather than “scars.” Clinically, as well as histologically, they differ considerably from involuted hemangioma. A subset of patients with KHE do not have an associated coagulopathy. Treatment of KHE is not standardized, and depends on the morbidity, location, and radiologic features. Multimodal therapy may include steroids, chemotherapy (most commonly vincristine), sirolimus, interferon, antifibrinolytic agents, antiplatelet agents, embolization, or radiation. Surgery may be considered for localized lesions; however, excision is often not an option due to diffuse intramuscular involvement. Studies are underway to compare different therapies.

Tufted angioma, first described in the late 1980s, is a benign vascular tumor typified by tufts of capillaries in the dermis. The clinical appearance ranges from erythematous, indurated, annular nodules to plaques, with or without hypertrichosis, commonly occurs on the trunk and extremities, and may be associated with Kasabach-Merritt phenomenon. KHE and tufted angioma may represent a continuum. Other vascular diagnoses which may have thrombocytopenia and gastrointestinal bleeding in the neonatal age group include multifocal lymphangioendotheliomatosis with thrombocytopenia (MLT), a rare disorder characterized by multiple red-brown macular lesions with positive lymphatic markers (LYVE-1, D2-40). Within the last several years, major research breakthroughs are unraveling potential etiologic factors leading to formation of hemangiomas, detailed in excellent reviews. As subtypes of hemangiomas with segmental cutaneous distribution and associated visceral anomalies became evident, researchers speculated involvement of neural-crest derived cells, further supported by identification of neural crest cell markers (neurotrophin receptor; p75) in proliferating hemangioma tissue. Several studies demonstrated markers for progenitor mesodermal stem cells (brachyury, GATA), or endothelial and hematopoietic cells (platelet endothelial adhesion molecule-1 (PECAM-1;CD31), intracellular adhesion molecule-2 (ICAM-3), bcl-2 gene expression, KDR +, CD133 +, CD34 + endothelial precursor cells, and lymphatic endothelial hyaluronan receptor-1, vWF;Snrk-1) in hemangioma tissue. Constitutive activation of the endothelial tie-2 receptor and VEGFR2-related signaling pathways has been identified in human hemangioma of infancy. Clonality of endothelial cells was demonstrated as the potential role of endothelial cells in hemangioma development. Bischoff et al. isolated hemangioma-derived stem cells, which unlike other precursor cells, grew in vitro and differentiated in vivo into cells with properties of hemangiomas including the eventual presence of adipocytes as seen in involuting hemangiomas. Hemangiomas and placental vessels express common proteins including GLUT-1 (glucose transporter-1). This discovery is of diagnostic utility and spearheaded insights into placenta-based hypotheses. For example, Mihm proposes a “metastatic niche” theory for hemangioma development, suggesting the placenta prepares hemangioma precursor cells which “home” to sites of hemangioma growth. Proliferating hemangiomas have been shown to express VEGF-A as well as genes involved with NF-kappa-B-related pathways. More recently, urinary excretion of MicroRNA126 was detected as a possible biomarker for infants with hemangiomas. Levels of this molecule correlated with hemangioma size and proliferative state and VEGF-A isoforms and pericyte expression of DLL4 has been documented. In addition, pro-apoptotic factors and appearance of adipocytes during the involution phase studies support a role for inflammation and immunoregulation in this process. Somatic mutations in GNAQ and GNA11 have been identified in RICH and NICH tissue samples.
Pyogenic granuloma (PG, also termed lobular capillary hemangioma) is an acquired vascular lesion of the skin and mucous membranes seen in pediatric patients. The lesions have a cervicofacial propensity but can also be located on the trunk or extremities. The majority occurs on the skin, and less frequently the mucous membranes (oral cavity and conjunctivae), and they may develop within capillary malformations (port wine stains). These lesions are small, papular, and tend to bleed. Treatment includes: topical β-blocker therapy, excision and linear closure, shave excision, cauterization, cryotherapy, CO 2 or pulsed dye laser, or sclerotherapy. Somatic RAS and BRAF mutations have been identified in PG.
KS is a neoplasm commonly but not exclusively seen in patients with AIDS (classic KS vs. AIDS-related KS). Post-transplant KS can occur as a result of immunosuppressive therapy for solid organ or hematopoietic cell transplantation. It is an unusual vascular neoplasm originally described in 1872. The clinical appearance begins as violaceous macular patches, which progress to plaques and papules and then nodules, which can extravasate. KS is thought to be multifocal rather than metastatic, with numerous lesions occurring simultaneously at different anatomic locations. Histologic features include spindle cells (derived from mesenchymal precursors), with rare mitotic figures. A novel human herpes virus, known as Kaposi sarcoma-associated herpes virus (KSHV), or human herpes virus type 8 (HHV8), has been identified in KS tissue, supporting a viral etiology. There is evidence that proliferation, inflammation, and angiogenesis occur in KS. One model suggests KSHV contributes to endothelial reinfection by inducing lytic gene expression and interfering with its DNA. Classic KS is more indolent than AIDS-related KS, which can be life-threatening and progress to visceral involvement. Therapies directed against KS include antiviral agents, antiangiogenic drugs, and immunosuppressive agents. Recent studies show the effectiveness of antiretroviral therapy suppressing HIV/AIDS-associated KS growth. Post-transplant KS has been shown to resolve with immune reconstitution and replacing calcineurin inhibitors with mTOR inhibitors.
Vascular malformations ( Tables 64.3 and 64.4 ) are present at birth and have no propensity to spontaneous involution (with rare exceptions). They are due to developmental anomalies of the vasculature and may involve one or several types of vessels (arteries, veins, capillaries, or lymphatics). They can be isolated lesions or syndromic, occurring in conjunction with other attributes ( Fig. 64.8 ). Vascular malformations are properly described according to the affected anomalous vascular channel and can range from capillary malformations (commonly referred to as port wine stains) ( Fig. 64.9 ) to large, bulky growths that can distort the normal structures of the body and potentially lead to a high output cardiac state (arterial malformations). Vascular malformations can progress with time. Many patients experience symptoms in relation to hormonal changes (e.g., puberty, menstrual cycle), and a subgroup of patients with vascular malformations may have recalcitrant disease (e.g., aggressive arteriovenous malformations [AVMs]). The pathologic basis of vascular malformations is a rich area of basic and genetic research. Several signaling pathways have been identified, leading to new therapies.
| Name | Features | OMIM |
|---|---|---|
| Blue rubber bleb nevus syndrome Bean syndrome TIE2 mutation somatic |
Multiple small soft venous malformations on skin, GI tract, elsewhere | 112200 |
| CLOVES syndrome PIK3CA somatic |
Congenital lipomatous overgrowth, vascular malformations, and epidermal nevi, skeletal/spinal anomalies | 612918 |
| Gorham syndrome Gorham Stout syndrome Cystic angiomatosis of bone, diffuse Disappearing bone disease |
Lymphangiomatosis, bony destruction | 123880 |
| Klippel Trénaunay syndrome PIK3CA somatic |
Capillary, venous, ± lymphatic malformation, hypertrophy of the related bones and soft tissues ± Atretic deep venous system of affected extremity |
149000 |
| Maffucci syndrome Osteochondromatosis/dyschondroplasia with vascular lesions IDH1, IDH2 somatic |
Enchondromatosis and subcutaneous spindle cell hemangioendotheliomas Risk of chondrosarcoma, other malignancies including CNS |
166000 |
| Proteus syndrome AKT1 somatic |
Gigantism, partial, of hands and feet, nevi, asymmetric and disproportionate overgrowth, hemihypertrophy, macrocephaly, dysregulated adipose tissue, vascular malformations. | 176920 |
| Capillary malformation-AVM ( Fig. 64.8 ) CMAVM1 5q14.3 RASA-1 loss of function CMAVM2 7q22.1 EPHB4 loss of function |
Multifocal small macular capillary malformations + AVM | 608354 |
| Parkes Weber syndrome 5q14.3 RASA-1 loss of function |
AV fistulae of extremity with soft tissue and skeletal hypertrophy | 608355 |
| Venous malformations, multiple cutaneous and mucosal; VMCM 9p21 TIE2/TEK gain of function AD Most are sporadic |
Focal venous dilation with sparse vascular smooth muscle cells Cutaneous, mucosal, ± underlying areas |
600195 |
| Hennekam syndrome 18q21.32 Type 1: CCBE1 Type 3: ADAMTS3 |
Intestinal lymphangiectasia, severe lymphedema mental retardation | 235510 |
| Hypotrichosis-lymphedema-telangiectasia syndrome HLTS 20q13.33 SOX18 |
Alopecia and/or areas of sparse hair, transparent skin, lymphedema, telangiectasia | 607823 |
| Lymphedema-distichiasis syndrome 16q24.3 AD or de novo FOXC2 loss of function |
Limb edema and double rows of eyelashes (distichiasis) ± other associated anomalies including cardiac, renal, vascular, CNS gene mutation | 153400 |
| Milroy disease 5q35.3 AD, AR, or de novo FLT4 VEGFR3 loss of function |
Primary congenital hereditary lymphedema type Ia | 153100 |
| Lymphedema praecox Meige disease Late-onset lymphedema |
Hereditary lymphedema type II Peri-pubertal onset |
153200 |
| Lymphangioleiomyomatosis 16p13.3, 9q34 |
Pulmonary (and extrapulmonary) lymphangiomyomatosis—female predominance, adult onset | 606690 |
| Hereditary hemorrhagic telangiectasia Osler Weber Rendu AD Loss of function HHT Type I 9q34.1 Endoglin Part of TGF-β receptor complex HHT Type 2 12q11-q14 ALK1/ACVRLK1 Cell-surface receptor for TGF-β superfamily HHT Type 3 5q31.3-q32 HHT Type 4 7p14 Juvenile polyposis/hereditary hemorrhagic telangiectasia syndrome; JPHT 18q21.1 SMAD4 Tumor suppressor; mutations affect TGF-β signaling |
Cutaneous, mucosal, and visceral telangiectasias and AVMs Epistaxis and gastrointestinal bleeding, ± pulmonary AV fistulas, hepatic, CNS, spinal AVM HHT1: cerebral AVMs > pulmonary AVMs HHT2: hepatic AVMs more common |
187300 600376 601101 |
| Cutis marmorata telangiectatica congenita CMTC Macrocephaly-cutis marmorata telangiectatica congenita |
Cutaneous reticulated mottling, telangiectasia, and phlebectasia, undergrowth or overgrowth of an involved extremity ± other anomalies | 219250 |
| Glomuvenous malformation GVM AD 1p22-p21 Glomulin FKBP (FK506 binding proteins)-associated protein, 48-KD; FAP48 |
Cutaneous venous malformations with glomus cells surrounding distended vein-like channels | 138000 |
| PHACES syndrome | P osterior fossa brain malformations Segmental facial H emangiomas A rterial anomalies C ardiac anomalies E ye abnormalities S ternal or midline anomalies |
606519 |
| Bannayan-Riley-Ruvalcaba 10q23.31 PTEN gene mutation Tumor suppressor |
Macrocephaly, multiple lipomas, vascular anomalies, pigmented macules of the penis | 153480 |
| Cowden syndrome 10q23.31 AD PTEN gene mutation Tumor suppressor PTEN hamartoma tumor syndrome (PHTS) |
Macrocephaly, multiple hamartomas, cutaneous verrucous lesions, gingival/buccal papules, facial trichilemmomas, risk of breast/thyroid/renal/endometrial malignancies, cerebelloparenchymal disorder VI (Lhermitte-Duclos disease) | 158350 |
| Solamen syndrome (type 2 Cowden’s) 10q23.31 biallelic AD with ?mosaic PTEN wild-type allelic loss PTEN gene mutation Tumor suppressor Overlap with proteus syndrome phenotype |
Segmental overgrowth lipomatosis Arteriovenous malformation Epidermal nevus Cancers—breast, thyroid, ovarian Loss-of-function PTEN activates AKT1 |
158350 |

It is essential to appreciate the normal development in the vasculature, which has two main components—(1) the closed circulatory system of arteries, veins, and capillaries, and (2) the unidirectional lymphatic system, which drains into the venous system (see Chapter 57 ). Each vascular branch has specified endothelial cells, designed to perform distinctive properties and functions, with characteristic morphologic features and marker expression. Mesenchyme-derived endothelial progenitor cells differentiate into arterial or venous networks. Lymphatic vessels are derived from venous- and non-venous mesenchymal-derived cells. Complex pathways involving cell-cell interactions modulated by transcription factors, growth factors, and environmental conditions generate the lymphatic and lymphatic vasculature. As vascular and lymphatic cells mature, they express different markers, which are exploited in research studies and clinical diagnosis. Disorders of vascular and lymphatic vessel morphogenesis may lead to vascular malformations (capillary, arterial, venous, lymphatic, or combined) lymphangiectasia, lymphatic anomalies with bony involvement (Gorham Stout/generalized lymphatic anomaly), or lymphedema (see Chapter 57 ).
Vascular malformations can be sporadic or inherited, and genetic mutations have now been identified for many of these (see below). Nonfamilial vascular malformations can be sporadic or due to somatic (post-zygotic) mutations, singularly expressed in a mosaic distribution in affected tissue. Inherited vascular malformations can be transmitted as autosomal recessive or autosomal dominant, or they can be genomic with a second somatic mutation within affected tissues, in which case carriers are unaffected. Inherited disorders can be identified via Sanger sequencing of blood samples, while samples of affected tissue are required to identify the causal mutation in somatic mosaic disorders. Happle proposed somatic mosaicism as a mechanism for survival of potentially “lethal genes” (i.e., germline expression would be fatal, thus they can only be conveyed mosaically), and he provides an updated review of this timely topic. Interestingly, most of the causal genes are mutations of TGF-β and phosphatidylinositol 3-kinase signaling pathways, not specific mutations of extracellular matrix or vascular structural proteins, even though the disorders affect specific vessels. Several publications summarizing genetic techniques and findings in vascular and lymphatic anomalies are now available. Studies on patient tissue, zebrafish, and transgenic mice have contributed to vascular and lymphatic vascular biology, providing models for study of specific disorders and potential therapies.
Several mutations have now been identified in patients with vascular anomalies. Familial vascular anomalies (caused by genomic mutations) include the following: hereditary hemorrhagic telangiectasia, familial mucocutaneous venous malformations, CM-AVM, glomuvenous malformation, PTEN Hamartoma syndrome, and heritable lymphedema syndromes.
Hereditary hemorrhagic telangiectasias (HHT) are caused by mutations in the TGFβ pathway/BMP/SMAD: Eng (endoglin), Alk1 (activin receptor kinase), and SMAD4 (mothers against decapentaplegic homolog 4). Tie-2 venous malformations are caused by activating mutations of the TEK receptor tyrosine kinase, which encodes protein tyrosine kinase Tie2. The ligand for this receptor is angiopoietin-1. Studies by Natynki et al. suggest that mutant TIE2 contributes to the endothelial morphology and coagulopathy seen in venous malformations due to upregulation of the MAPK pathway and plasminogen activator system, respectively. Two types of embryonic stem cells (outside and within the endothelium) have been identified in subcutaneous and intramuscular vascular malformations, with evidence to suggest that the endothelial population arises first, and may be affected by TIE2 mutations. Familial capillary malformation arteriovenous malformation may be associated with RASA1 (CM-AVM1) or EphrinB4 (CM-AVM2). RASA 1 mutations have also been identified in patients with Parkes Weber syndrome (OMIM 608355), a condition with multiple arteriovenous fistulas in an extremity, with skeletal and soft tissue hypertrophy.
Glomuvenous malformations (glomulin) are due to germline loss of function mutations in the glomulin gene, expressed in high penetrance. Cowden and Bannayan Riley Ruvalcaba syndromes harbor a germline mutation in the PTEN gene. Heritable lymphedemas may be due to a variety of mutations of genes important in lymphangiogenesis.
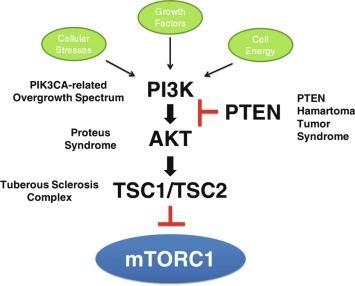
Sporadic and syndromic vascular malformations have been shown to be due to somatic mutations in an angiogenesis-related signaling pathway. Somatic mutations of TEK (TIE2) have been identified in sporadic venous malformations and blue rubber bleb nevus syndrome. GNAQ somatic mutations have been identified in capillary malformations/Sturge-Weber syndrome and congenital hemangioma. PI3K-AKT-MTOR-related disorders constitute a wide spectrum, including syndromic vascular anomalies and developmental brain disorders, termed PIK3CA-related overgrowth spectrum (PROS), PTEN mutations, and AKT mutations. Fig. 64.10 illustrates the PI3K-AKT-MTOR pathway. Proteus syndrome, CLOVES (congenital, lipomatous, overgrowth, vascular malformations, epidermal nevi, and spinal/skeletal anomalies and/or scoliosis) syndrome. Klippel Trénaunay syndrome, megalencephaly-capillary malformation (MCAP or M-CM), fibroadipose vascular anomaly as well as other mosaic overgrowth syndromes without vascular malformations are included in the PROS spectrum. Fig. 64.11 delineates the phenotypic criteria and clinical spectrum of PROS (PIK3CA-related overgrowth syndrome) disorders. Proteus syndrome is caused by a somatic AKT mutation, PTEN-related overgrowth syndromes are due to a germline PTEN mutation, and the remainder (CLOVES [congenital, lipomatous, overgrowth, vascular malformations, epidermal nevi, and spinal/skeletal anomalies and/or scoliosis]) syndrome is due to a somatic mutation of PIK3CA. Klippel Trénaunay syndrome, MCAP or M-CM, or fibroadipose vascular anomaly are due to somatic PIK3CA mutations. Recently, Couto et al. identified somatic mutations of mitogen activated protein kinase kinase 1 (MAP2K1) in most tissue samples from patients with extracranial arteriovenous malformations.

Disorders of the lymphatic circulation are common, diverse, and may have complex functional consequences ( Fig. 64.12 ). Clinical issues common to lymphatic anomalies reflect the tendency of these malformations to develop: (1) local (and systemic) infections/cellulitis (infectious and aseptic); (2) leakage (e.g., superficial blebs, chylous ascites, chylothorax, peritonitis, pleural effusions); (3) malabsorption syndromes with significant metabolic consequences; (4) craniofacial distortion interfering with swallowing, airway, or significant visceral dysfunction; (5) recurrences or complications after surgery; and (6) swelling of the affected anatomy, with functional limitation. More diffuse and aggressive lymphatic lesions may cause osteolysis (Gorham Stout disease), and generalized lymphatic anomaly is associated with multifocal lymphatic malformations ± bony involvement, without loss of cortical bone, as seen in Gorham Stout disease. Clinical and imaging features of Gorham Stout and generalized lymphatic anomalies are reviewed by Lala et al.

Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here