Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Fibrosis refers to the reorganization of loose extracellular matrix (ECM) and proliferation of fibroblasts that causes connective tissue thickening. Upregulation of profibrotic signaling pathways occurs in response to tissue destruction as a compensatory mechanism when parenchymal cells are unable to restore the normal structural integrity of affected organs. Recruitment of ECM proteins such as collagens, elastin, fibrillin, adhesive glycoproteins, integrins, and other secreted matricellular proteins is counterbalanced by activation of metalloproteinase enzymes that degrade ECM proteins to promote tissue remodeling and scar.
The functional consequences of fibrosis are diverse and hinge, in part, on the involved tissue subtype. For example, activation of preprogrammed mechanisms in response to dermal injury results in a highly coordinated series of molecular and histological events that causes the formation of a discrete collagen plug. In this scenario, fibrosis is physiological by generating an adaptive response to trauma that regulates hemostasis and decreases the probability of infection. By contrast, fibrosis is a pathogenic finding in numerous cardiovascular diseases, including idiopathic pulmonary arterial hypertension (PAH), atherosclerosis, systemic sclerosis, fibromuscular dysplasia, and hypertrophic cardiomyopathy, among many other examples. Pathogenic fibrosis is solely detrimental and therefore contradistinctive to maladaptive fibrosis, which may have favorable and unfavorable consequences. For example, replacement fibrosis following myocardial infarction maintains the structural integrity of the myocardium but also serves as a nidus for unstable reentry tachyarrhythmia and progression to heart failure.
Collagen quantity is often regarded as the standard for characterizing the pathogenicity of fibrosis. However, collagen content is an insufficient metric in isolation, as increased collagen is critical to physiological fibrosis, detrimental in pathogenic fibrosis, and of ambiguous relevance to benign tumors of fibroblast proliferation, such as keloid, and normal aging ( Fig. 9.1 ). Understanding the nuances of vascular fibrosis requires appreciation for its pathobiology, because classifying fibrosis based only on its distribution without regard to collagen biofunctionality is an incomplete paradigm. Similarly, as will be discussed in greater detail, traditional paradigms that ascribe global fibrosis to activation of a single master switch, particularly via transforming growth factor (TGF)-β signaling, may also fail to capture a wide range of important mechanisms that regulate ECM and collagen deposition related to end-organ injury in cardiovascular tissue. In turn, a contemporary understanding of the molecular mechanisms that regulate fibrosis is an important step toward potentially identifying novel treatment targets for fibrotic cardiovascular diseases.
The ECM is composed of water, proteins, glycosaminoglycans, and minerals but is acellular. Collagen is the principal structural protein of the ECM and accounts for approximately one-third of the total protein mass in mammals. There is disagreement on the optimal criteria for defining collagen: although all 28 isoform members of the collagen family are glycoproteins containing a triple helix, not all profibrotic triple helix proteins are identified as collagen, such as adiponectin. The stereometric conformation of collagen is a three parallel polypeptide motif: a polyproline II–type helical coil wrapped tightly forming a right-handed triple helix ( Fig. 9.2 ). ( 2S )-proline and 2 S ,4 R -4-hydroxyproline account for 28% and 38% of collagen amino acids, respectively ; every third residue is glycine, which by virtue of its smaller size and polarity, allows for tight packing of each strand that ultimately is the basis for the tensile strength of collagen (e.g., Gly-Xaa-Yaa). Thus the most common triplet sequence is glycine-proline-hydroxyproline (predicted denaturation enthalpy Δ G ° = −1.8 kcal/mol), resulting in a helical pitch of 7/2 (20.0 Å axial repeat).
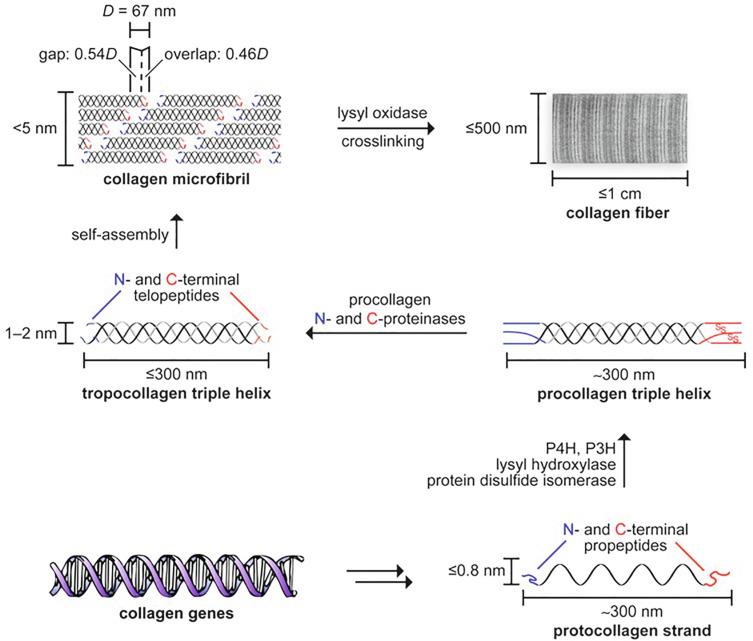
In general, unique collagen isoforms are identified with respect to their corresponding encoding gene (designated by a Roman numeral). Each of three polypeptide α-chains (662 to 3152 amino acids) is labeled by an Arabic numeral that indicates unique structural properties. Thus the Col2A1 gene encodes collagen II, which has three identical α-chains (homotrimer), designated as (α 1[II]) 3 . By contrast, the Col1A gene encodes the heterotrimer collagen I, which may have either two α1-chains and one α2-chain (α1[I] 2 , α2[I]), or, alternatively, three α1-chains (α1[I]) 3 ( Table 9.1 ).
| Collagen Type | α-Chains | Molecular Species |
|---|---|---|
| Collagen I | α1(I), α2(I) | (α1[I]) 2 , α2(I) |
| Collagen II | α1(II) | (α1[I]) 3 |
| Collagen III | α1(III) | (α1[III]) 3 |
| Collagen IV | α1(IV), α2(IV), α3(IV), α4(IV), α5(IV), α6(IV) | (α1[IV]) 2 , α2(IV) α3(IV), α4(IV), α5(IV) (α5[IV]) 2 , α6(IV) |
| Collagen V | α1(V), α 2(V), α 3(V), α 4(V) | (α1[V]) 2 , α2(V) (α1[V]) 3 (α1[V]) 2 , α4(V) α1(XI), α1(V), α3(XI) |
| Collagen VI | α1(VI), α2(VI), α3(VI), α4(VI), α5(VI)c, α6(V) |
|
| Collagen VII | α1(VII) | (α1[VII]) 3 |
| Collagen VIII | α1(VIII) | (α1[VIII]) 2 , α2(VIII) α1(VIII), (α2[VIII]) 2 (α 1[VIII]) 3 (α2[VIII]) 3 |
| Collagen IX | α1(IX), α2(IX), α3(IX) | (α1[IX], α2[X], α3[X]) |
| Collagen X | α1(X) | (α1[X]) 3 |
| Collagen XI | α1(XI), α2(XI), α3(XI) | α1(XI), α2(XI), α3(XI) α1(XI), α1(V), α3(XI) |
| Collagen XII | α1(XII) | (α1[XII]) 3 |
| Collagen XIII | α1(XIII) | (α1[XIII]) 3 |
| Collagen XIV | α1(XIV) | (α [XIV]) 3 |
| Collagen XV | α1(XV) | (α1[XV]) 3 |
| Collagen XVI | α1(XVI) | (α1[XVI]) 3 |
| Collagen XVII | α1(XVII) | (α [XVII]) 3 |
| Collagen XVIII | α1(XVIII) | (α1[XVIII]) 3 |
| Collagen XIX | α1(XIX) | (α1[XIX]) 3 |
| Collagen XX | α1(XX) | (α1[XX]) 3 |
Proline is derived from the amino acid L-glutamate and synthesized by cyclization of glutamate-5-semialdehyde into its biosynthetic precursor 1-pyrroline-5-carbyoxylic acid. Owing to its secondary amino group, proline may exist in the cis or trans conformation. However, the beneficial effect of proline on thermodynamic stability of collagen requires isomerization from the cis to trans orientation. Posttranslational modification of proline residues by prolyl 4-hydroxylase results in hydroxylation of its γ-carbon to form hydroxyproline, which provides a thermodynamic and mechanical advantage of this modification when in the Yaa position.
Fibrillar collagen refers to collagen subtypes that generate striated fibrils; specifically, type I-III, V, XI, XXIV, and XXVII. In human biology, fibrillar collagens constitute the majority of ECM. The tensile strength of an individual collagen fibril is high, ranging from 0.2 to 0.86 GPa, which suits its functionality as a tissue scaffold and anchor for matrix metalloproteinases (MMPs). The C -propeptides are enzymes that coordinate selection, alignment, and organization of α-chains, which is required for formation and elongation of the triple helix to generate procollagen in the endoplasmic reticulum. Next, the procollagen is transported in a coat protein II complex to the endoplasmic reticulum-Golgi apparatus, where it may be modified further by N- and C- proteinases to form fibrils. Ultimately, procollagen is transported from the Golgi apparatus to the extracellular space via a range of processes, including bulk flow, microtubule docking, and cell membrane fission. Stabilization of collagen fibrils is modulated by lysyl oxidase, which occurs via the formation of highly reactive aldehydes from lysine and subsequent interaction between reactive aldehyde pairs causing mechanical cross-links. The fundamental hallmarks of the fibrosis phenotype are increased cellular stiffness and impaired tissue compliance.
A wide range of perturbations to the vascular environment is associated with upregulation of collagen synthesis, ECM remodeling, and histopathological fibrosis. For example, vascular inflammation and thrombotic remodeling promote synthesis, release, and recruitment of growth factors, angiogenic simulators, and vasoactive cytokines. If unopposed or sustained, injury to the endothelial basement membrane occurs and results in the initiation of a larger cascade characterized by increased vascular permeability, platelet degranulation, and clot formation. Ultimately, activation of fibroblasts, myofibroblasts, macrophages, and endothelial or epithelial cell phenotype switching to profibrotic cells of mesenchymal origin (e.g., endothelial-mesenchymal transition) ensues and results in increased collagen protein synthesis.
Profibrotic signaling has been studied largely through activation of the TGF-β superfamily of cytokines. This includes various TGF-β isoforms, bone morphogenetic proteins, and growth and differentiation factors. Overactivation of canonical TGF-β signal transduction is complex and regulated by a wide spectrum of processes implicated in cardiovascular disease, including increased oxidant stress, necrosis, neurohumoral signaling (e.g., renin-angiotensin-aldosterone axis), and local hypoxia that stimulate TGF-β ligand binding to serine/threonine kinase type I or type II receptors (TGF-β-R1/2, respectively). Stimulation of the TGF-β-R1/2 complex results in phosphorylation of TGF-β-R2 by TGF-β-R1, which is required for downstream activation of various SMAD targets, particularly SMADs 3-5. Counteractivation of inhibitory SMADs, particularly SMADs 6 and 7, offsets nuclear accumulation of SMAD-DNA binding in the cell nucleus, preventing transcription of profibrotic genes, including connective tissue growth factor (CTGF). Under pathogenic circumstances, tonic upregulation of this pathway promotes excessive fibrillar collagen synthesis and deposition. In the case of heart failure, for example, increased circulating levels of angiotensin II (Ang II) activate TGF-βR1–dependent collagen synthesis in vascular smooth muscle cells (VSMCs) and left ventricular cardiomyocytes through a mechanism involving Erk1/2 signaling. Alternatively, Ang II also induces SMAD2 association with the CTGF promoter in VSMCs in vivo, supporting converging lines of evidence suggesting TGF-β–independent pathways also regulate vascular fibrosis.
Similarly, aldosterone promotes fibrosis, in part, by modulating the redox potential of vascular cells. Activation of NADPH oxidase activity by aldosterone has been shown to increase reactive oxygen species (ROS) generation in macrophages, systemic vascular endothelial cells, pulmonary artery endothelial cells (PAECs), and cardiomyocytes. Cultured proximal tubular cells treated with pathophysiologically relevant levels of aldosterone demonstrate increased mitochondrial ROS accumulation that induces epithelial-mesenchymal transition via ERK1/2 and p66Shc phosphorylation. Conversely, inhibition of the antioxidant enzyme glucose-6-phosphodehydrogenase (G6PD) by aldosterone perturbs the redox balance of vascular endothelial cells and results in diminished nitric oxide bioavailability and endothelial dysfunction. Aldosterone-induced oxidant stress, in turn, is associated with increased expression of plasminogen activator inhibitor (PAI)-1, CTGF, and galectin-3. These proinflammatory mediators regulate crosstalk with TGF-βR-1–dependent signaling, providing a molecular basis for overlap between inflammatory and fibrotic pathophenotypes commonly observed in cardiovascular diseases associated with increased aldosterone.
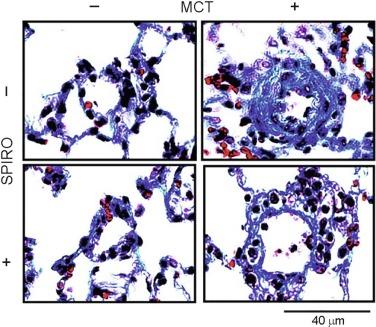
Importantly, the angiotensin type 1 (AT-1) receptor and mineralocorticoid receptor (MR) are important in mediating the genomic and profibrotic effects of Ang II and aldosterone, respectively. Thus these are bona fide treatment targets by which to prevent or attenuate cardiovascular fibrosis. For example, in experimental PAH, nonselective and selective MR antagonism with spironolactone and eplerenone, respectively, prevents or reverses vascular fibrosis of distal pulmonary arterioles ( Fig. 9.3 ). However, there are alternative stimuli that may directly or indirectly result in AT-1/MR-signal transduction, which are important potential treatment targets for cardiovascular fibrosis. The vasoactive peptide endothelin-1 (ET-1), for example, is increased in heart failure, systemic hypertension, and pulmonary vascular disease and is a potent stimulator of aldosterone synthesis in the zona glomerulosa of the adrenal gland and in pulmonary endothelium. In addition to its vasoconstrictor effects, ET-1 stimulates fibroblast activation, which is linked to cardiac fibrosis, and ET-1–CTGF signaling is reported in VSMCs via endothelin type-A receptor signaling.
Upregulation of noncanonical TGF-β signaling targets TRAF4, TRAF6, TAK1, MAPKs, ERK, NF-κB, PI3K-Akt, and JNK. These intermediaries are promiscuous and implicated in a wide range of adverse signaling cascades. However, several of these pathways have been studied in association with microRNA (miR) regulation of vascular fibrosis, particularly via miR130/301, miR-29a, and miR-181 a/b that target YAP/TAZ, collagen and elastin, and p27, respectively. A list of some profibrotic miRs and their putative targets are provided in Table 9.2 . The mechanisms by which these and other miRs regulate fibrosis are diverse but, importantly, include paracrine signaling. In particular, cardiac fibroblasts exert a range of transcellular communication capabilities through miR signaling involving exosomes, apoptotic bodies, and microvesicles, which are important for propagation of fibrotic remodeling beyond the site of initial cardiovascular injury.
| miRNA | Target | Mode of Action |
|---|---|---|
| miR-1 | Fibulin-1 | Delivery during left ventricular pressure overload attenuates fibrosis |
| miR-21 | Protein sprouty homologue 1, protein sprouty homologue 2, PTEN | Inhibits fibroblast apoptosis and increases fibroblast growth factor 2 secretion; leads to endothelial dedifferentiation |
| miR-26a | Collagen α-1(1) chain, connective tissue growth factor | Regulates nuclear factor κB and fibrosis development |
| miR-29 | Collagens, elastin, other matrix genes | Targets extracellular matrix genes |
| miR-34 | Vascular endothelial growth factor, neurogenic locus notch homologue protein 1, vinculin, PPP1R10 | Involved in cardiomyocyte aging; inhibits miR-34 and limits cardiac fibrosis |
| miR-101 | c-fos | Overexpression rescues pressure overload–induced fibrosis |
| miR-122 | Transforming growth factor-β1 | Reciprocally regulated in patients with aortic stenosis |
| miR-132 | Ras/Rap GTPase-activating protein SynGAP; methyl-CpG-binding domain protein 2 | Saphenous vein–derived pericyte progenitor cells mediate antifibrotic signaling in the infarcted heart |
| miR-133/miR-30 | Connective tissue growth factor | Involved in fibrosis development through connective tissue growth factor targeting |
| miR-133a | Collagen α-1(1) chain | Transgenic overexpression in cardiomyocytes prevents fibrosis development during pressure overload and diabetic cardiomyopathy |
| miR-155 | Unknown | A macrophage-derived miRNA involved in cardiac inflammation and fibrosis |
| miR-199b | Dual-specificity tyrosine-(Y)-phosphorylation regulated kinase 1A | Inhibition prevents cardiac fibrosis under left ventricular pressure overload |
| miR-208 | Myosin-6, myosin-7 | Inhibition leads to reduced fibrosis development under cardiac stress |
| miR-214 | Sodium/calcium exchanger 1 | Inhibition leads to excessive cardiac fibrosis development after myocardial infarction |
The MMP family consists of zinc-dependent endopeptidases that exert collagenolytic activity by virtue of site-specific hydrolysis. For collagen I, for example, MMP-2 and the two membrane-type MMP, MT-1-MMPs, target the Pro-Gln-Gly 775 ~ Ile 776 -Ala-Gly for cleavage. The final common pathways for degradation via hydrolysis involve proteolysis or urokinase plasminogen activator receptor–associated protein/Endo180–mediated endocytosis. In addition to the interaction between MMP and MT-MMP proteins with collagen, alternative pathways relevant to cardiovascular fibrosis for collagen catabolism have been reported, including α2β1 integrin–dependent phagocytosis in the lysosome. The initiation of collagen degradation generally involves mechanical strain on the fibrils, which facilitates their physical dissociation and breakdown. Although fibronectin, other α2β1-targeting proteins, and the tetraspanins CD63 and CD151 have been implicated in the initiation of collagen degradation, the factors that prompt these critical steps relevant to vascular fibrosis in cardiovascular diseases are not well characterized.
The ECM provides strength and structure to the myocardium and permits signal transduction among surrounding cells. Executing these functions is a dynamic process that involves continuous collagen deposition and breakdown. Cardiac fibroblasts secrete procollagen chains that organize into fibrils and undergo posttranslational cross-linking, which maintains tissue strength. Type I collagen comprises the majority of the ECM in the myocardium and provides strength, whereas type III collagen provides (recoil) elasticity. Pathological fibrosis arises when there is aberrant healing or response to injury in which collagen deposition exceeds clearance. Overall, myocardial fibrosis results in increased tissue stiffness, decreased cardiac compliance, and, ultimately, impaired contractility with increased susceptibility to malignant arrhythmias and sudden cardiac death.
Two characteristic patterns of fibrosis occur in the myocardium: replacement fibrosis and interstitial fibrosis. Replacement fibrosis occurs in response to injury in which myocytes die and are “replaced” by fibrotic scar. The most common example of replacement fibrosis occurs as a result of myocardial infarction and is observed in the blood flow distribution of the involved coronary artery. Additional myocardial diseases in which replacement fibrosis is observed include hypertrophic cardiomyopathy and dilated cardiomyopathy, which exhibit stereotypical fibrosis patterns ( Fig. 9.4 ).
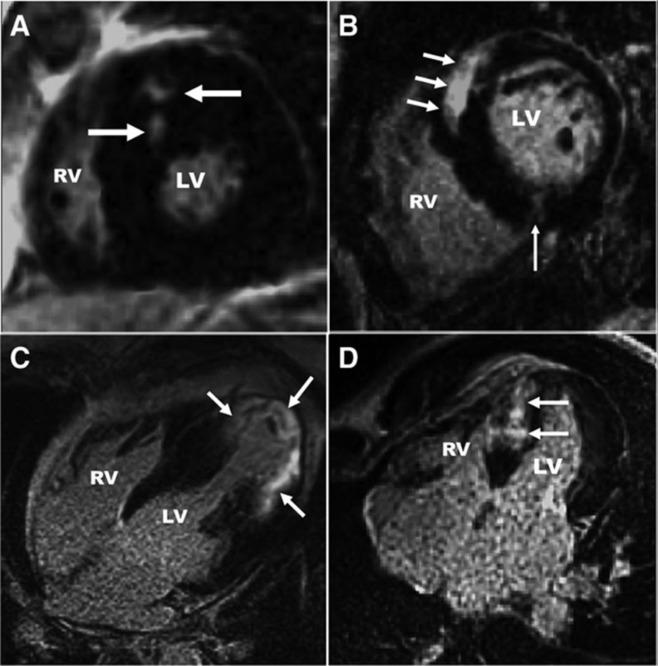
Interstitial fibrosis arises from excess collagen deposition by activated myocardial fibroblasts, known as myofibroblasts. The composition of interstitial fibrosis is disproportionately type I collagen, which increases collagen crosslinking leading to myocardial stiffness. The underlying triggers for fibroblast activation and the factors that contribute to individual variation in the development of interstitial fibrosis are unknown.
Processes implicated in interstitial fibrosis include inflammation, immune response, infection, and metabolic diseases, among others. Specific mediators of fibroblast activation, among many others, include TGF-β, tumor necrosis factor-α, CTGF, proteolytic enzymes, angiogenic factors, and miRNAs. The relative contributions of these molecular mediators and specific diseases in which they exert their effects are incompletely understood. Nonetheless, these factors represent potential targets to prevent or reverse myocardial fibrosis by virtue of their involvement in fibroblast activation. Interstitial fibrosis increases with age and is observed in patients with systemic hypertension, heart failure with preserved ejection fraction, and systemic sclerosis. Importantly, interstitial fibrosis is reversible and thus an important target for intervention to improve cardiovascular outcomes.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here