Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Labor results in significant pain for many women that is individualized, dynamic, and unpredictable.
Although the effects of obstetric analgesia and anesthesia on the fetus and neonate are typically benign, there is potential for adverse neonatal effects.
During pregnancy, labor, and delivery, women undergo fundamental changes in anatomy and physiology that affect all organ systems, significantly alter pharmacokinetic and pharmacodynamics responses to many drugs commonly used in anesthesia, and have important implication for anesthetic administration.
Opioids are commonly used systemic medications for labor and delivery but are administered with limitations on both dose and timing because they readily cross the placenta and are associated with a risk of neonatal respiratory depression in a dose-dependent fashion.
Neuraxial analgesia (epidural, spinal, combined spinal-epidural, and dural puncture epidural techniques) is the most widely used and most effective method for labor analgesia. Epidural labor analgesia is a catheter-based technique that provides continuous analgesia during labor using administration of medication into the epidural space. Neuraxial analgesia does not increase the risk for cesarean delivery, and with use of more current techniques is no longer associated with an increased use of instrumented vaginal delivery (forceps or vacuum).
For the patient without an epidural catheter, spinal anesthesia is the most common regional anesthetic technique used for cesarean delivery. Use of general anesthesia for cesarean delivery is typically reserved for situations where neuraxial anesthesia is contraindicated or emergent delivery is needed.
Labor results in significant pain for many women that is individualized, dynamic, and unpredictable. Modern techniques for labor analgesia and obstetric anesthesia, essential for operative and cesarean delivery, provide an effective and safe alternative to women seeking reduced pain with childbirth. Although the effects of obstetric analgesia and anesthesia on the fetus and neonate are typically benign, there is potential for adverse neonatal effects. This chapter introduces some of the scientific background and clinical techniques used in providing obstetric analgesia and anesthesia, as well as some of the potential maternal and neonatal complications.
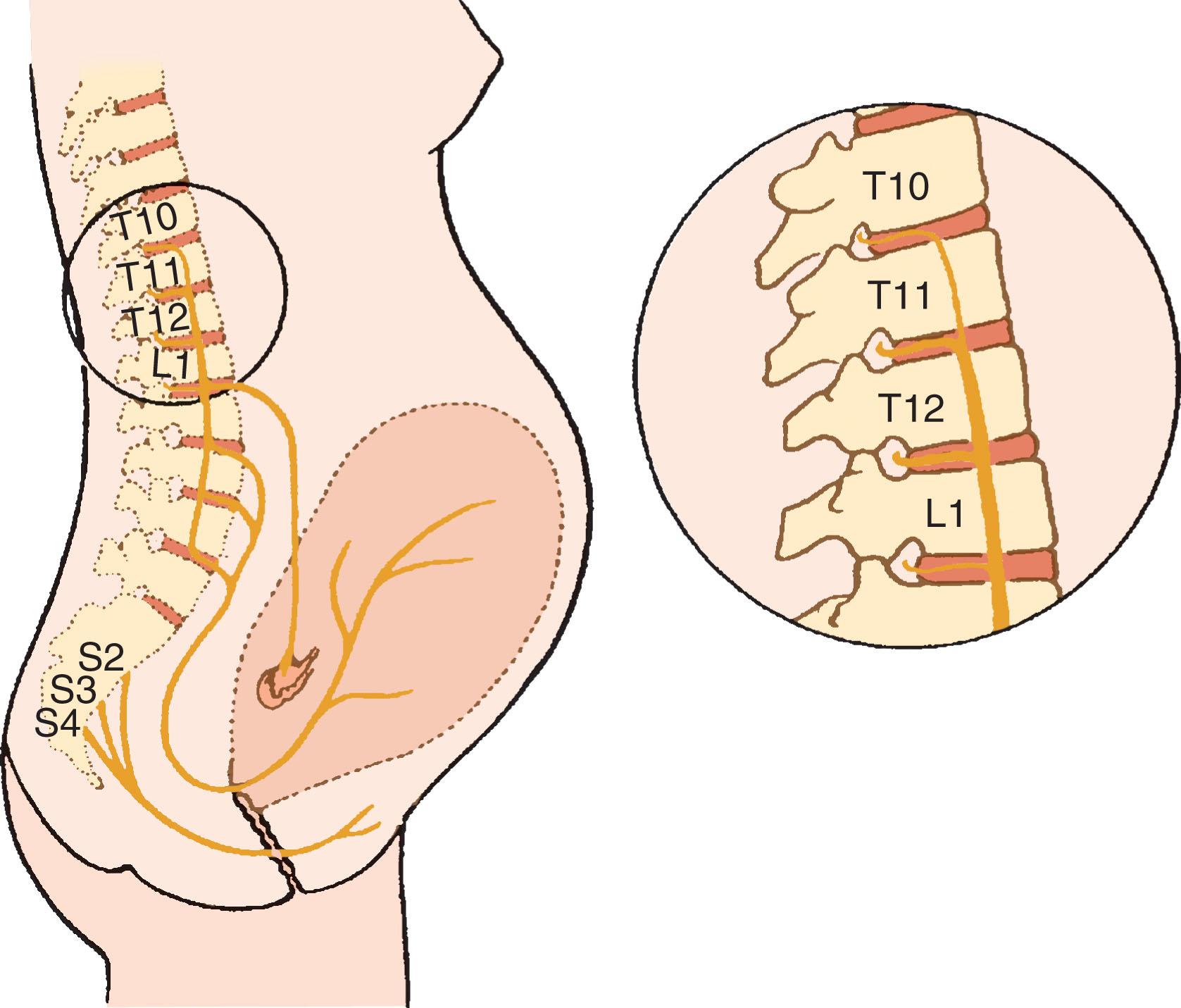
Labor is a continuous process divided into three stages. The first stage of labor begins with cervical dilation accompanied with regular uterine contractions. The second stage of labor begins when the cervix is fully dilated and concludes with the delivery of the neonate. The third stage ends with the delivery of the placenta. Contraction of the uterus, dilation of the cervix, and distention of the perineum cause pain during labor and delivery. Somatic and visceral afferent sensory fibers from the uterus and cervix travel with sympathetic nerve fibers to the spinal cord ( Fig. 14.1 ). These fibers pass through the paracervical tissue and course with the hypogastric nerves and the sympathetic chain to enter the spinal cord at T10 to L1. During the first stage of labor (cervical dilation), the majority of painful stimuli are the result of afferent nerve impulses from the lower uterine segment and cervix, as well as contributions from the uterine body causing visceral pain (poorly localized, diffuse, and usually described as “a dull but intense aching”). These nerve cell bodies are located in the dorsal root ganglia of levels T10 to L1. During the second stage of labor (pushing and expulsion), afferents innervating the vagina and perineum cause somatic pain (well localized and typically described as “sharp”). These somatic impulses travel primarily via the pudendal nerve to dorsal root ganglia of levels S2 to S4. Pain during this stage is caused by distention and tissue ischemia of the vagina, perineum, and pelvic floor muscles, associated with descent of the fetus into the pelvis and delivery. Neuraxial analgesic techniques that block levels T10 to L1 during the first stage of labor must be extended to include S2 to S4 for effective pain relief during the second stage of labor.
During pregnancy, labor, and delivery, women undergo fundamental changes in anatomy and physiology. These alterations are caused by changing hormonal activity, biochemical shifts associated with increasing metabolic demands of a growing fetus, placenta, and uterus, and mechanical displacement by an enlarging uterus.
Cardiac output increases during pregnancy, reaching an output 50% greater than the prepregnant state by the third trimester. The increase in cardiac output is due in part to both increases in heart rate (15% to 25%) and stroke volume (25% to 30%). During labor, maternal cardiac output increases during the first and second stages, reaching an additional 40% above prelabor values in the second stage. Each uterine contraction results in the auto-transfusion of 300 to 500 mL of blood back into the maternal central circulation. The greatest increase in cardiac output occurs immediately after delivery, when values can increase as much as 75% above predelivery levels. This abrupt increase in cardiac output is secondary to the loss of aortocaval compression, autotransfusion from the contracted uterus, and decreased venous pressure in the lower extremities.
Maternal systemic vascular resistance decreases 20% in normal pregnancy by term gestation. This results in a 5% to 20% decrease in systemic blood pressure starting near mid-gestation with diastolic pressures decreasing more than systolic. Reduced cardiac output and hypotension can occur when a pregnant woman is in the supine position because of aortocaval compression by the gravid uterus. Significant supine aortoiliac artery compression occurs in 15% to 20% of term pregnancies and vena caval compression is universal, often as early as 13 to 16 weeks gestation. Therefore, supine positioning is avoided during anesthetic administration in the second and third trimesters. Vena caval compression also contributes to lower extremity venous stasis and increased risk of thromboembolus. Significant lateral tilt is typically employed during cesarean delivery as 30 degrees is often required to relieve venacaval compression. This improves cardiac output and preserves uterine blood flow. Because neuraxial blockade can impair compensatory increases in sympathetic tone that facilitates maintenance of blood pressure during supine positioning, maternal tilt positioning is also frequently employed with administration of neuraxial labor analgesia to help preserve uterine blood flow.
Physiologic (dilutional) anemia of pregnancy occurs as a result of a greater increase in plasma volume (45% to 55%) than in red blood cell volume (20% to 30%) by term gestation. Average blood loss at delivery—approximately 500 mL for vaginal delivery and 1000 mL for cesarean delivery—is well tolerated because of this expanded blood volume and autotransfusion (about 500 mL) from the contracted uterus after delivery.
Beginning early in pregnancy, maternal capillaries become engorged causing mucosal edema and tissue friability throughout the pharynx, larynx, and trachea. These changes can make ventilation by mask, laryngoscopy, and tracheal intubation more challenging. In addition, the presence of comorbidities such as preeclampsia, upper respiratory tract infections, and active pushing with increased venous pressure during the second stage of labor further exacerbate airway tissue edema.
At term, minute ventilation is increased approximately 45% to 50%, as a result of an increase in tidal volume and a small increase in respiratory rate. In addition, oxygen consumption is increased by more than 20%, and functional residual capacity is decreased by 20%. The combination of these changes (increased oxygen consumption and decreased oxygen reserve) result in a state promoting rapid desaturation during periods of apnea. The changes in both airway and respiratory physiology during pregnancy make ventilation and intubation more difficult and increase the potential for complications. A multi-institutional database of adverse obstetric anesthesia events noted rates of failed maternal intubation for cesarean delivery were approximately 1:533, although none of these 10 failed obstetric intubations resulted in maternal mortality.
The gravid uterus increases intragastric pressure and causes the stomach and esophagus to reposition, resulting in decreased competence of the esophageal sphincter. Elevated progesterone and estrogen levels further reduce esophageal sphincter tone and placental gastrin decreases gastric pH. Consequently, most pregnant women experience symptoms of gastric reflux. Furthermore, gastric emptying is delayed by active labor, pain and administration of opioids. Delayed gastric emptying and decreased competence of the esophageal sphincter cause an increased risk of pulmonary aspiration with induction of general anesthesia, which has important implications for airway management that are discussed in detail in the General Anesthesia section.
Uterine weight and blood flow increase throughout gestation from approximately 100 mL/min before pregnancy to approximately 700 mL/min (10% of cardiac output) at term gestation, with 80% of the uterine blood flow perfusing the intervillous space (placenta) and 20% supporting the myometrium. Uterine vasculature has limited autoregulation and remains (essentially) maximally dilated under normal conditions during pregnancy.
Maternal uterine blood flow decreases as a result of either reduced uterine arterial perfusion pressure or increased arterial resistance. Decreased perfusion pressure can result from systemic hypotension secondary to reduced cardiac preload from hypovolemia, aortocaval compression, or significant decreases in vascular resistance from the initiation of neuraxial anesthesia or induction of general anesthesia. Uterine perfusion pressure can also decrease from increased uterine venous pressure associated with vena caval compression (e.g., supine position), uterine contractions (particularly during prolonged contractions and uterine tachysystole), or significant increase in intra-abdominal pressure (pushing during second stage or seizure activity). Despite these potential effects, phenylephrine (alpha-adrenergic) is useful for treating maternal hypotension secondary to neuraxial anesthesia, and it has been demonstrated to result in less fetal acidosis and base deficit compared to treatment with ephedrine (primarily beta-adrenergic) in many clinical trials. If treated promptly, transient maternal hypotension does not lead to fetal depression or neonatal morbidity. Additionally, phenylephrine is the vasopressor of choice for preventing and managing hypotension during cesarean delivery.
Exchange of drugs across the placenta occurs by passive diffusion, facilitated diffusion, transporter-mediated mechanisms, and vesicular transport. Most drugs less than 1000 Da molecular weight, if not ionized, cross the placenta by diffusion. The maternal drug concentration is usually the primary determinant of maternal-fetal transfer but maternal protein binding, molecular weight, lipid solubility, and drug ionization also contribute to maternal-fetal exchange. Although there is at least some placental transfer of most drugs, placental transfer of a variety of drugs is severely limited. During administration of anesthesia, drugs employed with significantly reduced placental transfer include succinylcholine, nondepolarizing muscle relaxants, heparin, and glycopyrrolate. Anesthetic drugs that readily cross the placenta include volatile anesthetic agents, opioids, and benzodiazepines. Local anesthetics and meperidine cross the placenta in a nonionized state, but once in the fetal circulation with a relatively lower pH, they become more ionized and can accumulate. (See the Neuraxial Local Anesthetics section.)
The pain of labor is highly variable and described by many women as severe. Factors influencing the patient’s perception of labor pain include duration of labor, maternal pelvic anatomy in relation to fetal size, use of oxytocin, parity, participation in childbirth preparation classes, fear and anxiety about childbirth, attitudes about and experience of pain, and coping mechanisms. The choice of analgesic method resides primarily with the patient. The medical condition of the parturient, stage of labor, urgency of delivery, condition of the fetus, and availability of qualified personnel are also factors. Many different techniques are available to alleviate labor and delivery pain, and none appears to increase the risk of cesarean delivery. Analgesia refers to pain relief without loss of consciousness; regional analgesia denotes partial sensory blockade in a specific area of the body, with or without partial motor blockade. Regional anesthesia is the loss of sensation, motor function, and reflex activity in a limited area of the body. General anesthesia results in the loss of consciousness, and the goals for providing general anesthesia typically include hypnosis, amnesia, analgesia, and skeletal muscle relaxation.
Techniques for labor analgesia must be safe for both mother and fetus and individualized to satisfy the analgesic requirement and desires of the parturient; they also must accommodate the changing nature of labor pain and the evolving, varied course of labor and delivery (e.g., spontaneous vaginal delivery, instrumentally assisted vaginal delivery, and cesarean delivery). The current approaches to pain relief are outlined in Box 14.1 .
Nonpharmacologic methods
Systemic opioids
Inhaled nitrous oxide
Regional techniques
Epidural
Spinal
Combined spinal-epidural
Dural puncture epidural
Paracervical block
Pudendal block
There are a variety of nonpharmacologic techniques for labor analgesia. Although many seem to reduce labor pain perception, most lack the rigorous scientific methodology for the useful comparison of these techniques to pharmacologic methods. Although data are limited, acupuncture, acupressure, transcutaneous electrical nerve stimulation, relaxation, and massage all demonstrate a modest analgesic benefit during labor. Other techniques such as hypnosis and intradermal water injections have not been shown to be more effective than placebo. A meta-analysis reviewing the effectiveness of a support individual (e.g., doula, friend, hospital staff, family member) noted that parturients with a support individual used fewer pharmacologic analgesia methods, had a decreased length of labor, experienced less dissatisfaction, had a lower incidence of operative or cesarean deliveries, and had improved 5-minute neonatal Apgar scores.
Opioids are the only commonly used systemic medications for labor and delivery, but are administered with limitations on both dose and timing because they readily cross the placenta and are associated with a risk of neonatal respiratory depression in a dose-dependent fashion. Although pain relief from the administration of systemic opioids is frequently inadequate for the duration of labor, this option can be beneficial for short-term analgesia, particularly in early labor. Opioids are inexpensive, easy to administer, and do not require a trained anesthesia provider. However, they have a high rate of maternal side effects (sedation, respiratory depression, dysphoria, nausea, pruritus), can decrease fetal heart rate variability and fetal movements, and carry a potential risk of neonatal respiratory depression and changes in neurobehavior. Systemic administration of opioids at doses that are safe for mother and newborn provides some labor pain relief, but do not have the analgesic efficacy of regional techniques. Systemic opioids are recommended for administration in the smallest doses possible with minimization of repeated dosing to reduce the accumulation of drug and metabolites in the fetus. Larger doses would risk excessive maternal sedation, maternal respiratory depression, loss of protective airway reflexes, newborn respiratory depression, and impairment of both early breastfeeding and newborn neurobehavior.
Opioids differ in pharmacokinetics, pharmacodynamics, method of elimination, and the presence of active metabolites, but all readily cross the placental barrier through passive diffusion. Systemic opioids are most useful for patients with minimal to moderate pain, precipitous labor, or contraindications to neuraxial blockade, such as a coagulopathy.
Meperidine remains the most widely used opioid worldwide for labor analgesia. Maternal half-life of meperidine is 2 to 3 hours, with the half-life in the fetus and newborn being significantly greater and more variable at values between 13 and 23 hours. In addition, meperidine is metabolized to an active metabolite (normeperidine) that has a longer maternal half-life of 13 to 23 hours and significantly accumulates in the fetus after repeated doses. With increased dosing and shortened time interval between dose and delivery, there is greater neonatal risk of lower Apgar scores and newborn oxygen saturations as well as prolonged time to sustained respiration, abnormal neurobehavior, and more difficulty initiating successful breastfeeding.
Morphine was used more frequently in the past but is rarely used today. Its onset of action is about 20 minutes with a prolonged duration of analgesia (3 to 4 hours). The half-life is longer in neonates compared with adults, and it produces significant maternal sedation. Although mostly metabolized into an inactive metabolite (morphine-3-glucuronide), about a third of administered morphine is transformed into an active metabolite (morphine-6-glucuronide) with significant analgesic properties. Obstetricians sometimes administer intramuscular morphine (10 to 15 mg) in combination with 25 mg of promethazine in latent labor to provide analgesia and facilitate rest. A recent prospective cohort study found that use of this “morphine sleep” did not impact the mode of delivery, maternal complications, or cause adverse neonatal outcomes.
Fentanyl is a synthetic opioid with a short duration of action (approximately 30 minutes), no active metabolites, and a ratio of fetal to maternal plasma concentrations of approximately 1:3. In small intravenous (IV) doses of 50 to 100 μg over an hour, there were no significant differences in Apgar scores, respiratory depression, or neurobehavior scoring compared with newborns of mothers who did not receive fentanyl. In addition, a comparison of equianalgesic doses of IV fentanyl compared with IV meperidine demonstrated a decreased frequency of maternal nausea, vomiting, and prolonged sedation in the fentanyl group. Furthermore, neonates whose mothers received meperidine required naloxone more often compared with the fentanyl-exposed infants. There was no difference in the neuroadaptive testing scores of the two groups of infants. Fentanyl administration by a patient-controlled analgesia (PCA) device is occasionally used during labor for patients with contraindications to neuraxial analgesia. In a retrospective study, neonatal depression correlated with maternal dose even when the fentanyl PCA was terminated approximately 20 minutes prior to birth.
Remifentanil, an ultra-short-acting opioid rapidly metabolized by nonspecific serum esterases, is significantly metabolized by the fetus, with umbilical artery–to–vein ratios of approximately 0.3. Remifentanil administered by a PCA device is an analgesic option for women who have contraindications to neuraxial blockade. The primary benefit of choosing this rapidly metabolized drug is to minimize opioid-related side effects on the neonate. Remifentanil can be used effectively for labor with PCA dosing, but it is difficult to achieve satisfactory analgesia without potential of significant maternal respiratory depression, requires more intensive monitoring and nursing, and is therefore typically reserved for patients with contraindications to epidural anesthesia. In a prospective randomized controlled trial comparing the effectiveness of epidural analgesia to a remifentanil PCA with optimized settings, epidural analgesia was significantly more effective than PCA for labor analgesia. In addition, they observed more sedation and oxygen desaturation during remifentanil analgesia, but there was no difference between groups in fetal and neonatal outcomes. A meta-analysis of randomized controlled trials comparing epidural analgesia with remifentanil PCA found improved pain scores among parturients randomized to epidural analgesia. A more recent equivalence trial performed between remifentanil PCA and epidural analgesia found remifentanil was inferior to epidural analgesia for satisfaction of pain relief and pain relief scores. A retrospective study comparing use of remifentanil and fentanyl PCA for labor analgesia found a greater rate of transient maternal desaturation with remifentanil, but use of fentanyl resulted in greater need for assisted neonatal ventilation or administration of supplemental oxygen.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here