Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Obesity is the most common nutritional disorder in the United States, and it directly or indirectly accounts for a significant portion of health-related expenses. Lifestyle change, antiobesity medications, and bariatric surgery are evidence-based treatment options, but each requires training and time to implement.
Obesity, which is a degree of excess adiposity that can predispose to adverse health consequences, is a disease of body weight regulation, much as diabetes is a disease of glucose regulation. Body mass index (BMI), calculated as weight in kilograms divided by height squared in meters, is the most commonly used screening tool to assess the health risks related to weight.
More than 1.9 billion adults worldwide are overweight, and over 650 million are obese. Obesity causes an estimated 4.7 million deaths worldwide. In the United States, over 70% of adults are overweight, and the prevalence of obesity is about 42% in women and 44% in men. BMI rises between ages 20 and 60 years and falls slightly thereafter. Severe obesity is inversely associated with urbanization, with a significantly greater prevalence of obesity and severe obesity among adults living in nonmetropolitan areas compared with large metropolitan statistical areas. Black American women and Mexican Americans of both sexes have the highest rates of overweight and obesity in the United States. Women in lower socioeconomic classes are much more likely than those in higher socioeconomic classes to be obese, an association that reduces, but does not eliminate, the racial differences in the prevalence of obesity. Whether the remaining racial differences in the prevalence of obesity are due to genetic, constitutional, or social and environmental factors is not yet known.
The health risks associated with increased adiposity increase continuously as BMI exceeds 25 ( Table 201-1 ). Lower BMI values of 23 to 24 are recommended for Asian populations, who are at risk for the typical metabolic complications of obesity at lower BMI and waist circumferences than are individuals of other ethnicities. Risks associated with being overweight are modest but are higher in the presence of central obesity. Some persons who are overweight (BMI of 25.0 to 29.9) may have increased muscle mass rather than increased body fat; these individuals are not difficult to identify clinically and do not have an elevated waist circumference. The prevalence of comorbid conditions and risk of new morbid conditions increase considerably at a BMI of more than 30, which is the definition of obesity. Obesity is divided into three classes, also depending on BMI (see Table 201-1 ).
| OBESITY CLASS | BMI (kg/m 2 ) | |
|---|---|---|
| Underweight | <18.5 | |
| Normal | 18.5-24.9 | |
| Overweight | 25.0-29.9 | |
| Obesity | I | 30.0-34.9 |
| Obesity | II | 35.0-39.9 |
| Extreme obesity | III | ≥40 |
Young adults, who accumulate greater exposure to metabolic and mechanical damage from being overweight or obese throughout their lives, are at increased risk for chronic health conditions such as coronary heart disease and type 2 diabetes mellitus. By comparison, the increase in mean BMI with age, though deleterious, is not as much of a threat to population health as is a similar increase in the BMI of younger populations.
The lowest mortality rates for young adults have historically been for a BMI in the normal range (20.0 to 24.9), whereas the BMI associated with the lowest mortality rates is somewhat above 25 kg/m 2 for adults in their 60 s and 70 s. Data also suggest that the optimal BMI may be about 27.0 for older adults given current life expectancy data.
Genetic and constitutional susceptibility to obesity are heavily influenced by the environment. Studies of twins adopted into different families indicate that within a given environment, a significant portion of the variation in weight is genetic. However, the growing prevalence of obesity is related to changes in environment, not human genetics.
Variants in the fat mass and obesity-associated (FTO) gene, which are relatively common (16% of adults are homozygous for the predisposing allele), account for only a few percent of the differences in body weight. Large genome-wide association studies have identified over 100 loci that associate with BMI, but together these genes account for less than 3% of the variance in BMI. The majority of the genes associated with BMI are expressed in the central nervous system, thereby suggesting effects on ingestive behavior. Genome-wide association studies have also identified about 50 loci that are associated with waist-to-hip ratio after adjusting for BMI, but these loci combined account for only 1 to 2% of the variance in waist-to-hip ratio. Most of these genes are preferentially expressed in adipose tissue, thereby implying that hereditary differences in body fat distribution are driven by genetic factors.
Genetic obesity is seen in a variety of childhood-onset conditions, including autosomal dominant Prader-Willi ( Chapter 214 ) and Albright hereditary osteodystrophy, as well as autosomal recessive Laurence-Moon-Biedl, Cohen, Carpenter, Alstrom, and Tubby syndromes. Virtually all monogenic obesity syndromes cause hyperphagia by disrupting parts of appetite regulation pathways. For example, children with homozygous or compound heterozygous loss-of-function mutations in the genes encoding leptin (LEP) and the leptin receptor (LEPR) develop intense hyperphagia that results in severe obesity. Congenital leptin deficiency results in undetectable serum leptin concentrations, whereas leptin concentrations are not unusually increased in those with LEPR deficiency. Approximately 5% of patients with severe, early-onset obesity have been reported to have heterozygous loss-of-function MC4R mutations, which appear to be inherited in a codominant manner. The features of MC4R deficiency include hyperphagia, hyperinsulinemia, and increased linear growth. Homozygous or compound heterozygous mutations in proopiomelanocortin (POMC) can result in hyperphagia and early-onset obesity, isolated adrenocorticotropic hormone (ACTH) deficiency (due to lack of adrenocorticotrophin), and hypopigmentation of skin and hair (due to lack of signaling through the melanocortin 1 receptor). Humans lacking prohormone convertase 1 also develop severe, early-onset obesity and ACTH deficiency due to impaired processing of POMC.
Environmental factors can result in long-term, epigenetic effects that affect body weight regulation and the susceptibility to obesity-related health problems. For example, undernutrition in the last trimester of pregnancy and in the early postnatal period decreases the risk of adult obesity, although the low birthweight associated with undernutrition (or smoking) in late pregnancy also increases the risk of adulthood hypertension, abnormal glucose tolerance, and cardiovascular disease. In contrast, undernutrition limited to the first two trimesters of pregnancy is associated with an increased probability of adult obesity. Infants of diabetic mothers tend to be fatter than those of nondiabetic mothers, and children of diabetic mothers have a greater prevalence of obesity when they are 5 to 19 years old, independent of whether their mother is obese. Finally, intrauterine exposure to the diabetic environment results in an increased risk of diabetes mellitus and obesity in the offspring.
Dramatic changes in the environment of developed and even developing countries over the past 50 years include reduced demands for physical activity and alterations in the food supply ( Table 201-2 ). These changes in the food supply help explain why the reduced energy expenditures by persons in the industrial and postindustrial eras have not been accompanied by a decrease in energy intake.
| DIETARY | ACTIVITY |
|---|---|
| ↑ Energy density of foods | ↑ Sedentary behavior |
| ↑ Portion size | ↓ Activities of daily living |
| ↑ Variety ∗ | ↓ Employment-related physical activity |
| ↑ Palatability | |
| ↑ Availability | |
| ↓ Cost | |
| ↑ Caloric beverages (sugar-sweetened beverages) |
The consumption of energy-dense foods results in greater energy intake because adults tend to respond to food volume rather than to the energy content. Many high-fat foods are also energy dense and contribute to weight gain. The consumption of sugar-sweetened beverages, such as soft drinks and fruit juices, is not accompanied by a decrease in food intake to offset the extra energy intake and promotes weight gain. The larger portion size of food and beverages also increases food intake. Food variety can also affect energy intake, and the ready availability of an increased variety of entrees, sweets, snacks, and carbohydrates is associated with an increase in food intake and body fatness. In contrast, an increase in the variety of vegetables available does not appear to increase energy intake and is not associated with increased body fatness. Other factors include the reduced costs of food, increased availability, and palatability. Psychological factors such as dietary restraint or disinhibition, as well as the social context in which the food is present, can influence how food properties affect energy intake.
Physical activity ( Chapter 14 ) levels have been decreasing in the United States and worldwide, as reduced activity at work is not replaced, on average, by increased leisure activity. The result is decreased energy expenditure, estimated to be over 100 calories/day, at a time when the food supply has led to sustained or increased caloric consumption. The amount of time spent in sedentary pursuits is associated with an increased risk of overweight and obesity, likely independent of participation in traditional exercise activities.
The typical U.S. adult will take in and expend approximately 2000 to 3000 kcal/day. Consistent errors of even 1% in excess food consumption can result in body fat gain of 25 to 30 pounds in 10 years, assuming no change in energy expenditure, so adults who do not gain weight must regulate their average energy balance with greater than 1% precision. This balanced regulation of energy intake and energy expenditure requires both conscious and unconscious processes.
The excess energy consumed by adults is generally stored as triglycerides in adipocytes. Humans continuously recruit new adipocytes from a large preadipocyte pool to replace dying adipocytes. Although the primary means by which abdominal adipose tissue mass expands is by increasing the size of existing fat cells (adipocyte hypertrophy), this process can store only a limited amount of fat. Adults who gain leg fat accumulate more rather than larger adipocytes on average, thereby resulting in a net increase in the number of adipocytes. Some adults recruit new adipocytes more readily than others, and their body fat is more related to adipocyte hyperplasia (increased fat cell number) than adipocyte hypertrophy. Adults who gain fat primarily via adipocyte hypertrophy are more likely to be insulin resistant.
In addition to its role in storing excess energy as triglyceride, adipose tissue secretes a large number of hormones (termed adipokines), the most prominent of which is leptin. Leptin has a role in regulating appetite, and very low leptin concentrations result in increased hunger. The other well-known adipokine is adiponectin, which is thought to have metabolic protective properties. The functions of scores of other adipokines have not yet been clearly identified in humans.
Signals can affect hunger, which is the compelling need or desire for food; satiation, which is the state of being satisfactorily full and unable to consume more; and satiety, which is the sense of no longer being hungry. The hypothalamus is a key component of the regulation of the intake of food ( E-Fig. 201-1 ). Hypothalamic arcuate nucleus neurons synthesize neuropeptide Y (NPY) and agouti-related protein (AgRP) and are activated under energy-deficit conditions by reduced levels of insulin and leptin, thereby resulting in the drive to eat (orexigenic response). When energy intake is sufficient, POMC neurons in the arcuate nucleus generate responses that reduce appetite (anorectic responses). The neurons that express POMC also express cocaine- and amphetamine-related transcript (CART). POMC is processed to the melanocortin (MSH) peptides alpha, beta, and gamma MSH, which are melanocortin 3 and 4 receptor (MC4R) agonists. These arcuate nucleus neurons project to MC4R-expressing neurons in the paraventricular nucleus, where activation of MC4R generates the anorectic response. In conditions of energy deficit, the MC4R receptor is inhibited by AgRP. Neurons in the paraventricular nucleus also express NPY receptors, which, when activated, cause hunger. The balance among these numerous signals plays an important role in the desire to eat. Perhaps the most obvious example of how this pathway can be disrupted is the increase in hunger in response to high-dose, exogenous corticosteroids; suppression of POMC neurons by corticosteroids results in unopposed activity of NPY on paraventricular nucleus neurons.
Satiety, which is the sense of no longer being hungry, is a complex set of postprandial events that affect the interval to and/or the amount consumed at the next meal. The leptin receptor is highly expressed in the hypothalamus. When it is activated by leptin, which is secreted by adipocytes, the expression of POMC increases, thereby leading to reduced food intake. Gut peptides that can influence food intake are secreted in response to mechanical stimuli (e.g., the fullness of the stomach) and/or the presence of nutrients in the jejunum and ileum. These peptides include peptide YY 36 (PYY), glucagon-like peptide 1 (GLP-1), and oxyntomodulin (OXM) that, like leptin and insulin, inhibit neurons that express NPY and AgRP. In contrast, ghrelin activates NPY and AgRP neurons, thereby generating a sensation of hunger, an effect that is exacerbated by prolonged fasting, possibly because leptin secretion decreases out of proportion to the loss of body fat.
In addition to the hypothalamic energy balance system, other central nervous system responses have important effects on food intake ( E-Table 201-1 ). Relevant structures include the ventral striatum, where the reward aspects of food intake are determined; the prefrontal cortex, where executive function and impulse control take place; the brain stem, where vagal inputs from the gastrointestinal tract modulate food intake; and the cortex, where the social meaning of eating is interpreted. Other factors that have been shown to affect food intake include cholecystokinin, which causes satiation but has no effect on satiety, and pancreas-derived amylin, which acts on the gastrointestinal tract and the hypothalamus to inhibit appetite.
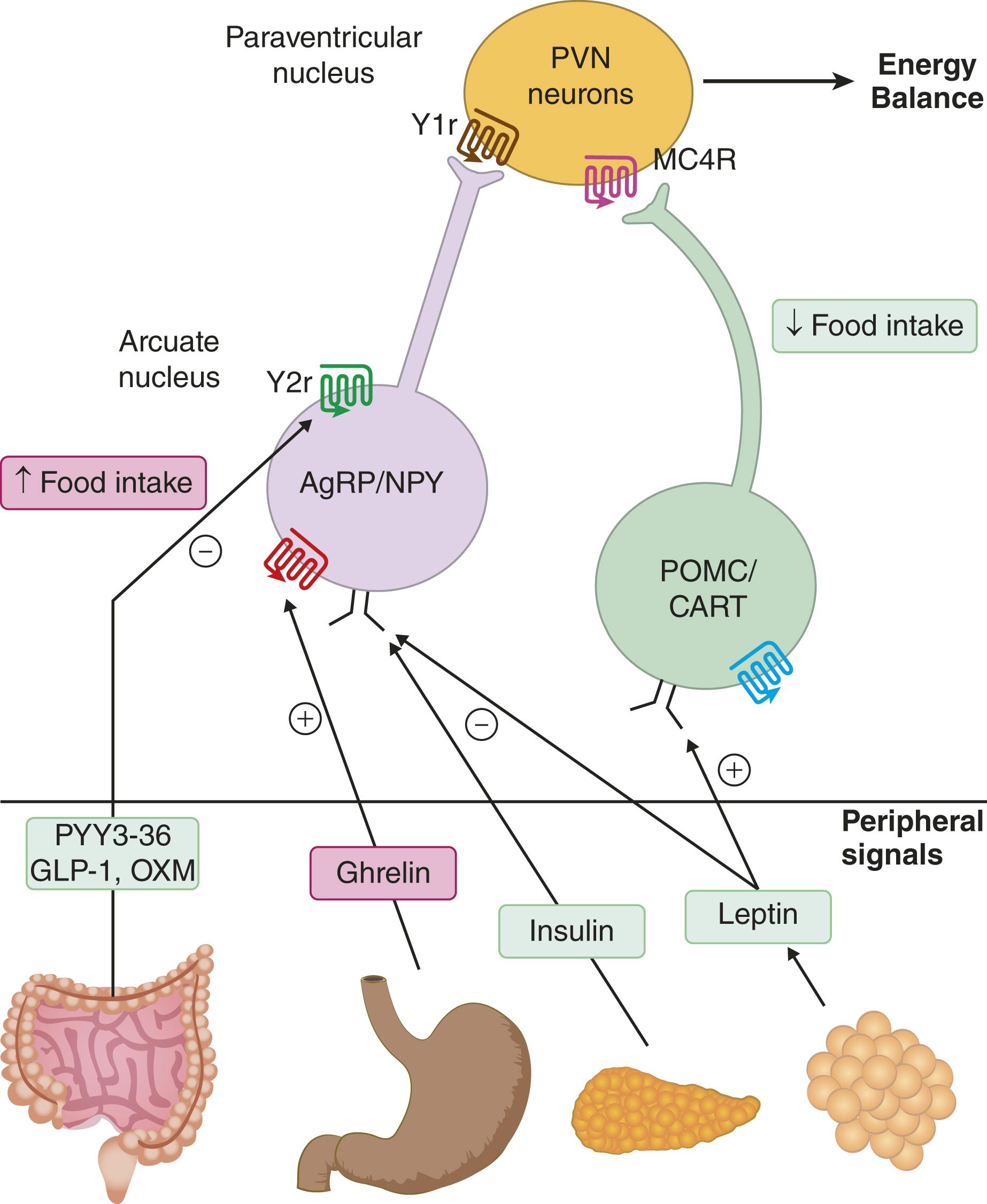
Signals of energy availability from the small intestine, stomach, pancreas, and adipose tissue are relayed to the hypothalamus via the circulation. In the arcuate nucleus of the hypothalamus, neurons that express NPY and AgRP are activated under energy deficit conditions and drive feeding (orexigenic) responses by signaling to NPY1 receptor (Y1r) neurons in the paraventricular nucleus (PVN). Ghrelin activates NPY and AgRP neurons, whereas PYY, GLP-1, OXM, insulin, and leptin inhibit these neurons. In the arcuate nucleus of the hypothalamus, neurons that express POMC generate anorectic signals under conditions of energy surplus. POMC neurons coexpress CART. Anorectic peptides (PYY, GLP-1, and OXM) activate POMC neurons. In the paraventricular nucleus, melanocortin melanocyte-stimulating hormone (α-MSH), which is a product of POMC cleavage, activates the MC4R, which generates a feeling of satiety. Depending on energy needs versus availability, the balance between activating NPY/AgRP as compared with POMC/CART neurons is thought to drive hunger and satiety.
| Brain region | Functions | Neurotransmitters |
| Hypothalamus | Energy homeostasis | Norepinephrine, GABA, POMC, CART, AgRP, NPY |
| Ventral striatum | Reward aspects of food intake | Dopamine |
| Prefrontal cortex | Executive function, impulse control | Norepinephrine, dopamine, glutamate |
| Brainstem, vagus nerve | Signals from GI tract | GLP-1 |
| Cortex | Social meaning of eating |
Daily energy expenditures in adults can range from less than 1400 kcal/day to more than 5000 kcal/day, with larger, more physically active individuals having the greatest energy needs. Typically, daily energy expenditure is divided into resting (or basal) metabolic rate, the thermic effect of food, and physical activity energy expenditure.
The basal metabolic rate (BMR) is the energy expenditure of lying still, at rest, awake, and in the fasting state. Most formulas calculate BMR as strictly measured, and BMR is considered the foundation of daily energy expenditure. Under most clinical circumstances, however, the resting metabolic rate (RMR) is measured because of the difficulty in measuring BMR; RMR is not necessarily measured before arising from bed and may not be in the fasting state, so it may be greater than BMR. For most sedentary adult Americans, BMR represents the major portion of energy expended during the day and may range from less than 1200 to more than 3000 kcal/day. Most (~80%) of the variability in BMR can be explained by the amount of lean and fat tissue. In addition, BMR is slightly lower in women than in men and in older than in younger adults even after accounting for the amount of lean and fat tissue. Heritable factors may account for as much as 10% of the interindividual differences in BMR. With an energy-restricted diet, BMR may decline out of proportion to the loss of fat-free mass. Reductions in the production of triiodothyronine from thyroxine and the sympathetic nervous system drive are thought to contribute to this phenomenon. Likewise, during brief periods of overfeeding, BMR increases slightly above that which would be expected for the amount of lean tissue present. Formulas to estimate BMR (available through numerous online calculators: https://www.calculators.org/health/bmr.php .) on the basis of height, weight, age, and sex are accurate to within 10% in approximately 90% of adults with BMIs of 18.5 to 45 kg/m 2 .
Muscle accounts for only 25% of BMR but can account for 80 to 90% of energy expenditure during exercise. Adipose tissue requires only about 3 kcal/kg of body fat per day, so it is a minor contributor to daily energy expenditure.
Brown fat is adipose tissue that expresses large amounts of uncoupling protein-1 (a protein that allows a mitochondrial membrane proton leak). When activated, this process results in the release of heat as opposed to chemical work from adenosine triphosphate—“uncoupling” of substrate oxidation from chemical or mechanical work. This thermogenic tissue, which exists only in small amounts in adults, might become a target for future interventions to reduce adiposity. Methods used to detect brown fat largely rely on 18 F-fluorodeoxyglucose positron emission tomography scanning of humans exposed to cold.
The energy expenditure of physical activity is a product of the amount of work done and the work efficiency of the individual. Work units are expressed as metabolic equivalents (METs; see Table 14-1 ), a multiple of the RMR. If an individual’s RMR is 1 kcal/minute (1440 kcal/day), a workload of 5 METs would be 5 kcal/minute. Although most sedentary individuals can work for only a limited amount of time at relatively low workloads, highly trained athletes can work at extremely high METs (>16) for extended periods.
Nonexercise activity thermogenesis is the energy expense of performing all activities other than exercise- or employment-related activity. Such activities may include tasks as mundane as getting up to walk 50 feet to the water fountain or spending 8 hours cleaning one’s living space. The range of observed nonexercise activity thermogenesis varies from less than 100 to more than 800 kcal/day. On average, about 10% of the energy content of food (ranging from a low of about 3% to a high of about 15%) is expended in the process of digestion, absorption, and metabolism of nutrients. The energy expended from a physically demanding job or volitional exercise may or may not be offset by reductions in spontaneous (nonemployment) activity.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here