Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The practice of surgical neuropathology can be challenging for the generalist and specialist alike. Much of the difficulty results from the intrinsic complexity of the human central nervous system (CNS), an organ that is unrivaled in regional variation and specialized organization. Nevertheless, a basic understanding of the types of cells and their regional organization in the normal brain is necessary for the practice of surgical neuropathology, because recognition of the abnormal rests upon a firm knowledge of the normal. Even in the current age of advanced neuroimaging and image-guided biopsies, many neurosurgically sampled specimens contain normal or only slightly abnormal tissue that needs to be recognized as nondiagnostic. Indeed, much of the anxiety that arises at the time of frozen section seems to be due to a discomfort with distinguishing normal from abnormal rather than from correctly categorizing an abnormal biopsy. The “sea of pink” noted under the microscope from a brain biopsy of normal brain tissue has been known to cause even the most experienced surgical pathologist a great degree of uncertainty. The changes of “reactive gliosis” only exacerbate the problem. Much like the pattern recognition approach used in this textbook for classifying diseases of the CNS, so too can the normal histology of the brain be approached based on recognition of tissue pattern. Like other forms of pattern recognition, this takes practice. For the neuropathologist, repeated exposure to normal CNS structure in surgical specimens is supplemented by the regular review of autopsy brains. For generalists that practice surgical neuropathology, review of autopsy brain sections can similarly add confidence. This chapter introduces the cell types and normal histology of the human CNS at a depth necessary for routine diagnostic practice. Most of its content describes the normal adult brain, but certain aspects of age-related phenomena and developmental considerations that are routinely encountered in diagnostic neuropathology are also considered.
Given the high degree of functional complexity, it may be surprising that the brain and spinal parenchyma consist predominantly of only two cell types, neurons and glia, while their coverings mostly feature meningothelial and mesenchymal cell types. The normal anatomy and histology of the pineal gland, pituitary gland, and peripheral nervous system are also covered to some extent in their respective tumor chapters. Both neurons and glia are large families with many members that have highly specialized functions, yet the underlying structure and cell biology of each retain some central features. Most imposing for the practicing surgical pathologist is the great degree of morphologic and geographic diversity of normal, reactive, and degenerative states of these two cell families.
Neurons are the sensing, integrating, transmitting, and effecting cells of the nervous system, communicating by chemical and electrical means. The spectrum of their morphology, connectivity, and function is enormous. As a rule, neurons have a cell body (i.e., perikaryon), branching processes called dendrites for integrating incoming signals, and a longer cell process—the axon—with a terminal synapse for chemically transmitting an electrical signal over a short space from one neuron to the next (or to a muscle cell through a neuromuscular junction). Cell body shape and size, as well as the number and arrangement of branching processes, vary considerably. For practicing pathologists, recognizing the major forms of neurons within their anatomic setting is requisite, because individual populations show differential vulnerability to injury and variable pathologic reactions in specific disease processes.
The pyramidal neurons of the cerebral cortex and subfields of the hippocampus represent a morphologic prototype ( Fig. 2.1A and B ). They are characterized by large, triangular cell bodies, a prominent apical dendrite extending toward the brain's surface, and numerous finer branching basal dendrites. Measuring approximately 10 to 50 microns in greatest dimension, their cell bodies contain abundant cytoplasm, variable hematoxyphilic Nissl substance (rough endoplasmic reticulum) near the entry zone of cell processes, and a large nucleus with open chromatin and a prominent nucleolus. Open chromatin and prominent nucleoli are typical of neurons that are almost constantly transcriptionally and translationally active and distinguish them from the more quiescent glia.
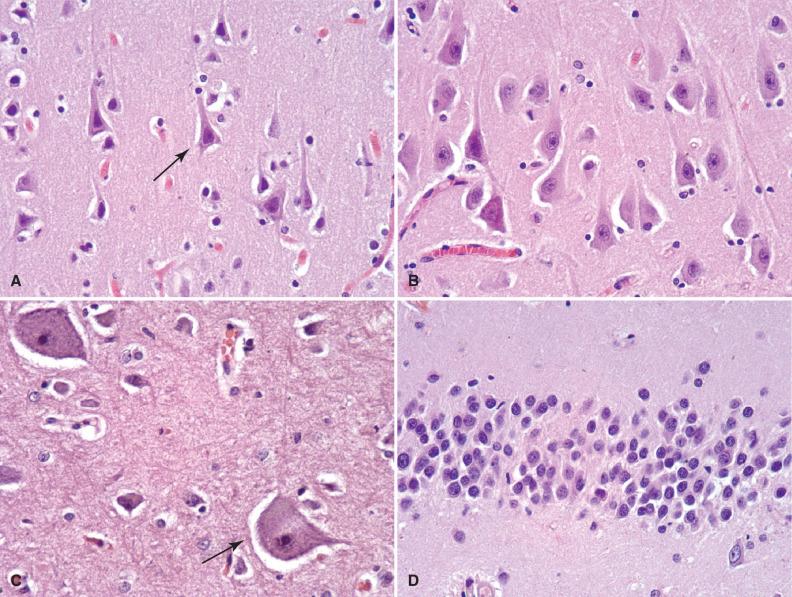
Cortical granular (stellate) neurons are the smaller counterparts of pyramidal neurons in the cortex, typically measuring 15 microns or less in diameter. Being interneurons, they have numerous shorter processes that remain within the confines of the cortex.
Betz cells are the largest neurons of the cerebral cortex (70 to 100 microns) and are found in the primary motor cortex where they dwarf their neighboring cortical pyramidal cells ( Fig. 2.1C ). Not only the cell size and amount of cytoplasm, but also the amount of Nissl substance and the number of visible processes far exceed normal pyramidal cells. Betz cells are upper motor neurons.
Small, tightly packed granular neurons form the stratum granulosum of the dentate gyrus in the medial temporal lobe, intimately connected to the hippocampus proper ( Fig. 2.1D ). These neurons are nearly as small as the cerebellar granular cells (see discussion to come) and have an extensive dendritic arbor that forms the adjacent molecular layer of the dentate gyrus.
Purkinje cells are large (50 to 80 microns), histologically distinctive neurons of the cerebellum with cell bodies that sit at the interface of the molecular and internal granular cell layers ( Fig. 2.1E ). Each neuron has a prominent pink cell body and an expansive dendritic tree with thick processes that extend into the molecular layer, as well as a large axon that travels centrally out of the cerebellar cortex.
Granular neurons of the cerebellar granular cell layer are tiny and densely packed, often displaying a linear arrangement or loose rosettes around delicate neuropil ( Fig. 2.1E ). Perinuclear cytoplasm is sparse, giving the appearance of only nuclei on hematoxylin-eosin (H & E) stains. This population can cause confusion on frozen section or cytologic preparations, because they resemble “small round blue cell tumors.”
Anterior horn cells are large, lower motor neurons (alpha motor neurons) that populate all levels of the spinal cord in the anterior horns and send long axonal processes via the anterior roots for their eventual termination on peripheral skeletal muscle endplates ( Fig. 2.1F ).
The CNS also contains a small number of highly specialized nuclei that contain neurons that produce specific bioaminergic neurotransmitters and project diffusely throughout the brain to affect global or regional tone. Rarely seen in biopsied material, these include the substantia nigra, locus ceruleus, raphe nuclei, and nucleus basalis of Meynert. The dopaminergic cells of the substantia nigra pars reticulata (and the ventral tegmental area) are large, heavily pigmented neurons with “ neuromelanin ” (not to be confused with melanin of melanocytes), which accumulates in the cytoplasm as coarse brown granules and represents a combination of oxidized and polymerized dopamine within lysosomal granules ( Fig. 2.1G ; selectively vulnerable in Parkinson disease). Similarly, the locus ceruleus, located near the fourth ventricle in the rostral pontine tegmentum, contains a population of large, pigmented neurons, which serve as a major source of norepinephrine in the brain (selectively vulnerable in Parkinson disease). Neurons located in the raphe nuclei, located along the midline of the brainstem, are similar in size and shape to the noradrenergic neurons of the locus ceruleus, but lack the pigmentation. These cells produce serotonin and have diffuse projections throughout the nervous system, but most heavily innervate limbic and sensory regions. Within the basal forebrain, inferior to the anterior commissure in a region called the substantia innominata, is the nucleus basalis of Meynert, a collection of large cholinergic neurons that project throughout the cerebral cortex (selectively vulnerable in Alzheimer disease).
Glia account for approximately 90% of all CNS cells and have been generally regarded as the “glue,” providing structural and functional support for neuronal elements. In fact, they are functionally much more diverse and biologically important than originally suspected, such that neurobiologists have shifted away from this overly “neuronocentric” perspective. Glia are divided into the macroglia or true glia—astrocytes, oligodendrocytes, and ependyma—and the microglia, which are actually of hematopoietic rather than true glial derivation.
Astrocytes are the multipolar, “star-like” glial cells of the CNS ( Figs. 2.2 and 2.3 ). They can be subdivided into protoplasmic and fibrillary families based on their location and morphology. Protoplasmic astrocytes reside in the cortex; fibrillary astrocytes populate the white matter. In addition to similar cell shapes and numerous processes, all astrocytes contain abundant cytoplasmic intermediate filaments, largely composed of glial fibrillary acidic protein (GFAP). In the resting state, astrocyte nuclei are recognized on H & E stained sections, but the scant, delicate cytoplasm and processes are not readily seen because they blend with surrounding neuropil. Nuclei are oblong with a chromatin pattern that is lighter and looser than either oligodendrocytes or neoplastic astrocytes ( Fig. 2.2A ). Nucleoli are not present in most resting astrocytes, in contrast to neurons. Many astrocytes have processes that terminate as end-feet on blood vessel walls, where they contribute to the blood-brain barrier. Others have processes that extend end-feet to the pial surface of the brain, contributing to the glia limitans of the brain-CSF barrier.
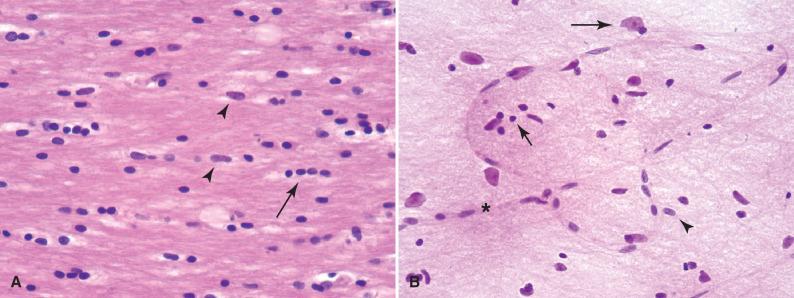
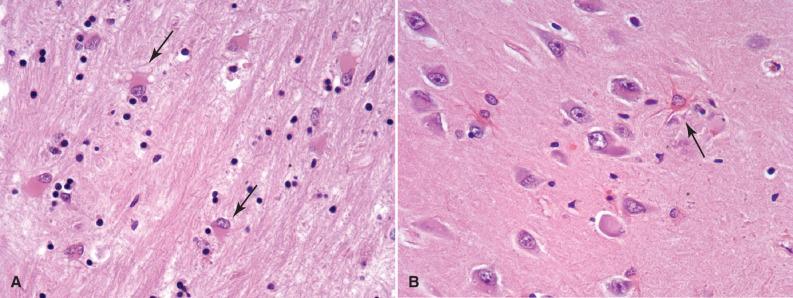
Astrocytes are activated in response to a variety of pathologic conditions (see Fig. 2.3 ). The morphologic spectrum of reactive astrocytosis is critical to recognize because (1) it focuses attention on pathologically affected regions for further evaluation; (2) it serves as a validation that a disease process is present in the CNS (i.e., rather than artifact); and (3) it often causes diagnostic dilemmas due to its morphologic overlap with neoplastic conditions. Reactive astrocytosis involves both proliferation and hypertrophy of astrocytes and its appearance varies with the chronicity and severity of insult. The initial response to insult is an overall hypertrophy, including enlargement of the cell body and processes, together with an activated appearance of the nucleus, with dispersion of chromatin and enlargement of nucleoli. In H & E stained sections, the presence of abundant astrocytic cytoplasm and visible cellular processes is almost always a pathologic finding ( Fig. 2.3B ). Immunohistochemistry for GFAP will highlight the reactive nature of these astrocytes, demonstrating mostly even spacing of cells with extensive arborizing of their processes and the orientation of the reactive cells to the underlying injury ( Fig. 2.3C ). Reactive astrocytes of longer duration often take on a gemistocytic (from the Greek word gemistos for “stuffed”) appearance, with large amounts of brightly eosinophilic cytoplasm in their eccentrically placed cell bodies ( Fig. 2.3A ). The relatively even spacing of these astrocytes and their radially arranged processes, respecting each other's territorial boundaries, help to distinguish them as reactive, rather than neoplastic.
Chronic reactive astrocytosis occurs around or within a slow-growing lesion and is often more fibrillar in nature, with numerous long astrocytic processes forming a layer of dense gliosis. Rosenthal fibers are large, flame-shaped or globular proteinaceous deposits that may be seen in this type of long-standing process; when present, this form of reaction is often termed piloid gliosis, due to its morphologic overlap with the compact regions of pilocytic astrocytoma ( Fig. 2.3D and E ). It is most often encountered adjacent to slow-growing neoplasms (e.g., craniopharyngioma, ependymoma, hemangioblastoma) and benign cystic lesions (e.g., pineal cyst, spinal syrinx).
Alzheimer type II astrocytes are a reactive form seen in states of elevated blood ammonia, usually related to renal or hepatic disease ( Fig. 2.3F ). They are present in highest concentration in the basal ganglia where cells show nuclear swelling, marked chromatin clearing, and micronucleoli. Cytoplasmic hypertrophy is not prominent in this form of astrocytosis and, usually, the nuclei have no appreciable cytoplasm.
Bergmann glia are specialized astrocytes located between the molecular and granular layers of the cerebellum. Cells are only one to two layers thick and can go unnoticed in resting states. In response to cerebellar injury, especially to individual Purkinje cell loss from ischemia or hypoxia, the reactive proliferation of this cell layer is referred to as Bergmann gliosis; on H & E stained sections, there is a replacement of Purkinje cells with one to three layers of oval nuclei associated with coarse GFAP-positive fibrillary processes radiating toward the pia ( Fig. 2.3G ).
Creutzfeldt cells are another form of reactive astrocytes that have abundant cytoplasm, the fragmenting of nuclear material giving the impression of multiple micronuclei ( Fig. 2.3H ). A related finding of “ granular mitoses ” in these reactive astrocytes may mimic proliferating astrocytoma cells [see Chapter 24 discussion of demyelinating diseases and tumefactive multiple sclerosis (MS)]. These cells are not specific, but are seen most often in active inflammatory diseases (classic in demyelinating disease).
Markedly enlarged and cytologically atypical astrocytes can be seen in many non-neoplastic conditions, although the nuclear atypia is often most pronounced in reactions to radiation ( radiation atypia; see Chapter 21 ) and progressive multifocal leukoencephalopathy (PML; see Chapters 23 and 24 ). In both conditions, nuclei can be bizarre with marked hyperchromasia, multilobation, and irregular outlines. The context of additional microscopic changes and the clinical history are often required to avoid a misdiagnosis of neoplasm.
Oligodendrocytes are the myelinating cells of the CNS and are therefore more numerous in white than in gray matter (see Fig. 2.2 ). With their thin, short cellular processes extending in all directions, oligodendrocytes provide internodes of myelination to multiple axonal processes in their environment. In H & E stained sections, only the nucleus of oligodendrocytes is usually visible due to the blending of cellular processes with the neuropil. A clear zone surrounding the nucleus, the so-called perinuclear halo, often highlights oligodendrocytes as well as tumors with similar cytologic features (i.e., oligodendrogliomas); in either case, this appears due to a retraction artifact of formalin fixation. Nuclei are generally round and regular, but vary from small and darkly basophilic (accounting for a majority) to slightly larger with pale vesicular nuclei. Nucleoli are not usually noted by standard light microscopy. In the white matter, oligodendrocytes are disposed along the length of axonal processes, whereas in the cerebral cortex, they are scattered within the neuropil and concentrated immediately surrounding neuronal cell bodies (satellite cells). In the latter location, they may serve as a progenitor population.
Ependyma are cuboidal to columnar epithelioid glial cells that form a single-layered covering of the ventricular system ( Fig. 2.4 ). On their ventricular (apical) surface, ependyma have microscopically visible cilia and microvilli, while their lateral surfaces are tethered to one another by desmosomes, forming a functional CSF-brain barrier. Ependymal cytoplasm is pale to eosinophilic, and nuclei are oval and hyperchromatic. Within the supratentorial and infratentorial compartments, ependyma are fairly homogeneous, varying slightly by anatomic location in their cell height and degree of ciliation ( Fig. 2.4C and D ). Within the spine, the central canal is lined by ependyma and serves as a conduit for CSF during childhood. In adulthood, the central canal is collapsed and vestigial, remaining only as a central collection of clustered ependyma throughout the spinal cord length ( Fig. 2.4E ). Along the lateral ventricles, especially posteriorly, it is fairly common to encounter either entrapped outpouchings of ependyma or small clusters forming canals ( Fig. 2.4F ). These do not represent hamartomas or malformations, but only remnants of imperfect development that are clinically inconsequential.
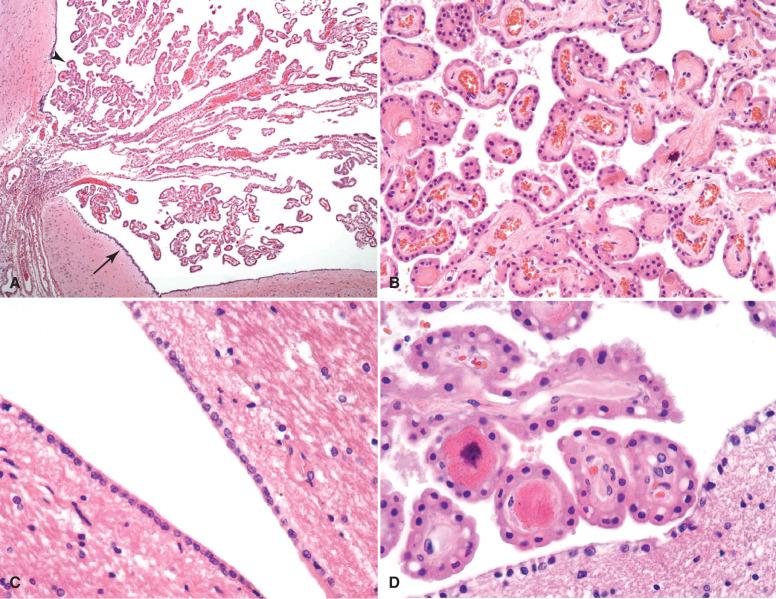
The choroid plexus is a functionally differentiated region of ependyma that extends into the ventricular space as frond-like tufts of epithelium that secrete the ultrafiltrate of CSF (see Fig. 2.4 ). Individual cells are found as a single layer upon a fibrovascular core. Compared to ependyma, they have larger, cobblestone-shaped cell bodies and contain small, bland, basally located nuclei. Microvilli extend from the apical surface. Tight junctions and desmosomes are present between choroid plexus cells to assure a viable blood-CSF barrier.
Microglia are small, elongated cells located throughout the CNS gray and white matter ( Fig. 2.5 ). In the resting state, microglia are easily overlooked because of their small size and bland appearance, yet they account for nearly 20% of the cellular population. In standard H & E sections, the nuclei of activated microglia are long, thin, and dark—leading to their designation as “rod-cells”—but their cytoplasm is difficult to visualize. Special stains based on silver carbonate or lectins provide contrast to small processes and delicate branches that extend from their tips. Microglia are not neuroepithelial in origin, but rather are derived from a monocyte/macrophage lineage that incorporates into the CNS early in development. Once established, they serve as antigen-presenting cells for immune surveillance and participate in inflammatory responses, particularly against viral pathogens.

Upon activation, microglia proliferate and migrate to sites of damage, and in this state (“microgliosis”) cells are more readily identified. Activation also causes increased expression of proteins such as major histocompatibility complex (MHC) I and II, which can be detected immunohistochemically. When microglia and astrocytes aggregate around a central focus of injury, such as a viral-infected neuron, they form a microglial nodule (see Chapter 23 ).
Another population of monocyte/macrophage–derived cells resides in the perivascular compartment, between the outer basement membrane of the vessel and the glia limitans. In distinction to parenchymal microglia, these perivascular macrophages are in continuity with the circulating monocyte population. Both perivascular and circulating populations of monocytes are recruited into the CNS parenchyma in response to severe injury, where they differentiate into tissue macrophages, often with foamy clear cytoplasm (i.e., gitter cells ) in order to perform phagocytic and immunologic functions. They are sometimes referred to as the “garbage collectors” of the CNS, because they clean up all necrotic debris, metabolic by-products, and foreign material.
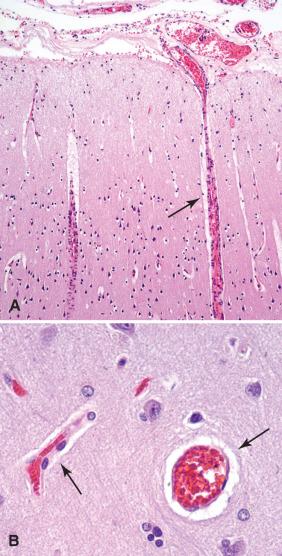
Similar to other organs, the brain has a population of vascular and perivascular cells essential for its oxygen and nutrient supply. Compared to their extracranial counterparts, the large arteries that run within the subarachnoid space have thinner muscular walls, have less adventitia, and lack external elastic lamina ( Fig. 2.6 ). As they penetrate into the brain parenchyma, larger arteries retain both a thin covering by the pia-arachnoid and a perivascular space, the Virchow-Robin space, which represents a continuation of the subarachnoid space, although its function and content remain controversial. No such space exists once the vessels become small capillaries and the endothelium is intimately associated with neuropil. Capillaries are formed by individual endothelial cells forming delicate tubular structures with widths suitable only for passage of individual circulating blood cells. The physiologically critical blood-brain barrier is formed predominantly by the specialized nature of CNS endothelial cell junctions and cannot be identified histologically. In particular, these endothelial cells lack fenestrations between them and are joined by specialized tight junctions that functionally preclude the free movement of substances between vascular and CNS spaces. Astrocytic end-feet and basal lamina elements further contribute to the integrity of this blood-brain barrier.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here