Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The entire cardiac output passes through the pulmonary circulation, so the lungs act as a filter, preventing emboli from passing to the left side of the circulation.
The lungs constitute a huge interface between the outside environment and the body, requiring the presence of multiple systems for defence against inhaled biological and chemical hazards.
In the pulmonary circulation there is active uptake and metabolism of many endogenous compounds, including amines, peptides and eicosanoids.
The lungs’ primary purpose is gas exchange, and to achieve this with such efficiency almost the entire blood volume passes through the lungs during each circulation. This characteristic makes the lungs ideally suited to undertake many other important functions. The location of the lungs within the circulatory system is ideal for its role as a filter to protect the systemic circulation, not only from particulate matter but also from a wide range of chemical substances that undergo removal or biotransformation in the pulmonary circulation. The pulmonary arterial tree is well adapted for the reception of emboli without resultant infarction, and the very large area of vascular endothelium gives the lung a metabolic role out of proportion to its total mass. This large interface between the external atmosphere and the circulation is not without its own hazards, and the lung must protect the circulation from many potentially harmful inhaled substances.
Sitting astride the whole output of the right ventricle, the lung is ideally situated to filter out particulate matter from the systemic venous return. Without such a filter, there would be a constant risk of particulate matter entering the arterial system, where the coronary and cerebral circulations are particularly vulnerable to damaging emboli. The vast majority of emboli are thrombus, but nonthrombotic emboli also occur and may be organic (e.g., air, tumour, fat, amniotic fluid) or inorganic (e.g., catheter tips or radiotherapy seeds).
Pulmonary capillaries have a diameter of approximately 7 µm, but this does not appear to be the effective pore size of the pulmonary circulation when considered as a filter. For example, it is well known that small quantities of gas and fat emboli may gain access to the systemic circulation in patients without obvious intracardiac shunting. Emboli may bypass the alveoli via some of the precapillary anastomoses that are known to exist in the pulmonary circulation, either between the bronchial and pulmonary circulations (page 73) or through the intrapulmonary arteriovenous anastomoses. The last of these are much larger than normal capillaries, at 25 to 50 µm diameter, but fortunately remain closed most of the time (page 12), most commonly opening during hypoxic conditions. Despite this, in one-third of normal healthy subjects echocardiography demonstrates transpulmonary passage of microbubble contrast, and this is reduced by breathing 100% oxygen. More extensive invasion of the systemic circulation may occur in the presence of a right-to-left intracardiac shunt, which is now known to be quite common. Postmortem studies show that over 25% of the population have a ‘probe-patent’ foramen ovale, usually in the form of a slit-like defect that acts as a valve, and which is therefore normally kept closed by the left atrial pressure being slightly greater than the right. In 10% of normal subjects, a simple Valsalva manoeuvre or cough results in easily demonstrable blood flow between the right and left atria. Paradoxical embolism may therefore result from a relative increase in right atrial pressure caused by physiological events or pulmonary embolism ( Chapter 29 ).
As far as the survival of the lung is concerned, the geometry of the pulmonary microcirculation is particularly well adapted to maintaining alveolar perfusion in the face of quite large degrees of embolization. However, a significant degree of embolization inevitably blocks the circulation to parts of the lung, disturbing the balance between ventilation and perfusion. This situation is considered in Chapter 29 . Pulmonary microembolism with small clumps of fibrin and/or platelets will not have a direct effect on gas exchange until it is very extensive. Plugging of pulmonary capillaries by microemboli does, however, initiate neutrophil activation in the area, leading to an increase in endothelial permeability and alveolar oedema, and has been implicated in the aetiology of acute lung injury ( Chapter 31 ).
Thrombi are cleared more rapidly from the lungs than from other organs. The lung possesses well-developed proteolytic systems not confined to the removal of fibrin. Pulmonary endothelium is known to be rich in plasmin activator, which converts plasminogen into plasmin, which in turn converts fibrin into fibrin degradation products. However, the lung is also rich in thromboplastin, which converts prothrombin to thrombin. To complicate the position further, the lung is a particularly rich source of heparin, and bovine lung is used in its commercial preparation. The lung can thus produce high concentrations of substances necessary for promoting or delaying blood clotting, and also for fibrinolysis. Apart from the lung’s ability to clear itself of thromboemboli, these substances may play a role in controlling the overall coagulability of the blood.
The skin, gastrointestinal tract and lungs form the major interfaces between the outside world and the carefully controlled internal body systems. Efficient gas exchange in the lung requires a physically very thin interface between air and blood, which leaves the lung vulnerable to invasion by many airborne hazards, both chemical and biological. These are almost entirely prevented from reaching the distal airways by the airway lining fluid found throughout the tracheobronchial tree.
Within the airway lining fluid there are two distinct layers, a periciliary or ‘sol’ layer which is of low viscosity containing water and solutes and in which the cilia are embedded, and a mucous or ‘gel’ layer above. ,
Large airways are completely lined by a mucous layer, whereas in smaller, more distal airways the mucus is found in ‘islands’, and a mucous layer is absent in small bronchioles and beyond. Mucus is 97% water, and about one-third of the remaining 3% is glycoproteins called mucins, which determine the viscoelastic and other properties of the mucus. The human genome encodes 17 mucins, of which only seven are secreted, while the remainder are membrane-bound mucins. Mucin is released by rapid (<150 ms) exocytosis from the mucous-secreting goblet cells in response to a range of stimuli, including direct chemical irritation, inflammatory cytokines and neuronal stimulation, predominantly by cholinergic nerves. Mucins have a core composed of glycoprotein subunits joined by disulphide bonds, and their length may extend up to 6 µm. The core is 80% glycosylated, with side chains attached via O -glycosidic bonds. Almost all terminate in sialic acid and possess microorganism binding sites. Mucus plays a vital role in pathogen entrapment and removal, and also has a variety of antimicrobial actions (see later).
The mucous layer is propelled cephalad by the ciliated epithelial cells ( Fig. 11.1 ) at an average rate of 4 mm.min −1 , to be passed through the posterior commissure of the larynx between the vocal folds into the oesophagus and swallowed, or removed by expectoration. The cilia beat mostly within the low-viscosity periciliary layer of airway lining fluid, with the cilia tips intermittently gripping the underside of the mucous layer, propelling the mucous layer along the airway wall.
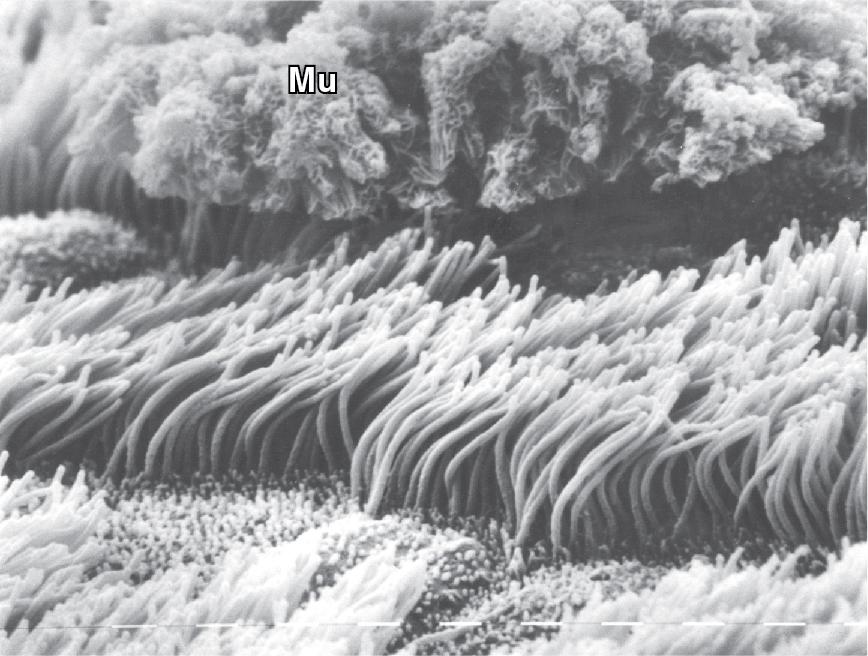
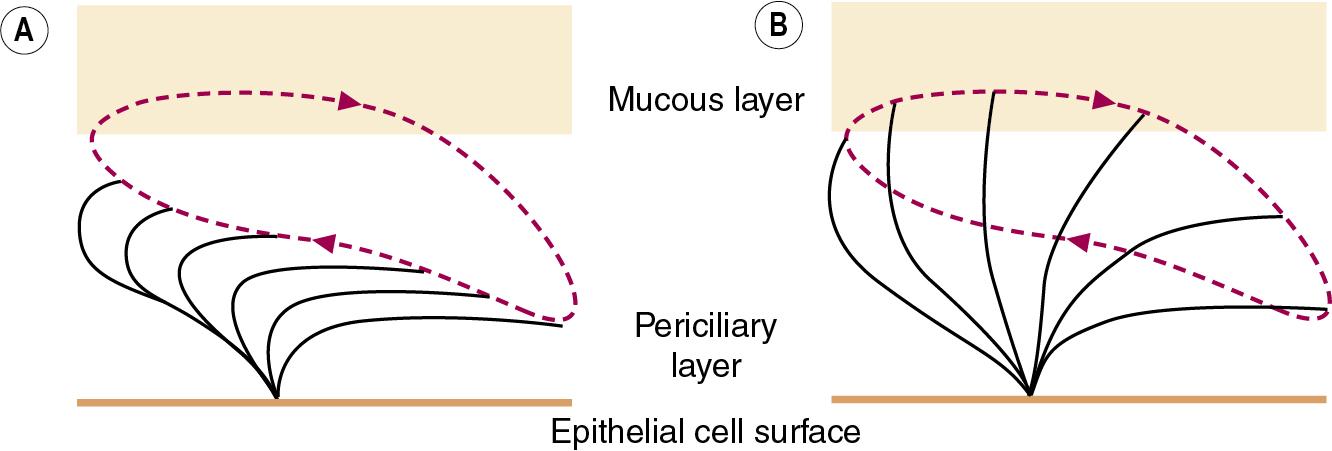
Cilial beat frequency is 12 to 14 beats per second, but this may speed up in response to increased extracellular adenosine triphosphate (ATP) levels, and is affected by pollutants, tobacco smoke, anaesthetic agents and infection. There is a general slowing of ciliary activity with increasing age. Two phases occur for each beat ( Fig. 11.2 ). First is the recovery stroke, which occupies 75% of the time of each cycle and involves a slow bowing movement away from the resting position by a sideways action of the cilium. Then follows the power stroke, in which the cilium extends to its full height, gripping the mucous layer above with claws on its tip, before moving forward in a plane perpendicular to the cell below and returning to its resting position. Adjacent cilia somehow coordinate their strokes to produce waves of activity that move the mucous layer along, probably by a physical effect of cilia stimulating adjacent cilia during the sideways sweep of the recovery stroke.
For this propulsion system to work effectively, it is crucial that the depth of the periciliary fluid layer be closely controlled to 7 µm deep, particularly considering the increasing amount of mucus that will occur each time two smaller airways converge into one larger airway. The depth of both layers of the airway lining fluid is controlled by changes in the volume of secretions and the speed of their reabsorption, with both processes occurring simultaneously in different regions of airways. If the periciliary layer reduces in depth, the gel layer will compensate for this by donating liquid to the periciliary layer to maintain the correct depth of fluid, an effect probably mediated by simple osmotic gradients between the two layers. The mucous layer may donate fluid to the periciliary layer until its volume is diminished by 70%. The reverse happens as the mucus converges on the larger airways, with the mucous layer absorbing excess periciliary water. Airway epithelium is freely permeable to water, so the volume of periciliary fluid is therefore determined by its salt concentration, which is in turn controlled by active ion transport on the surface of the epithelial cells. Several ion channels are responsible for this active control, the most important of which are amiloride-sensitive epithelial sodium channels and the chloride channel better known as the cystic fibrosis transmembrane regulator (CFTR) protein. CFTR is likely to be partially active at rest but is stimulated when sodium channels are inhibited. The factors responsible for controlling this system are incompletely understood, but a critical component is the various adenine nucleotides released from ciliated cells in response to mechanical stress, acting on the same cells to influence the ion channels controlling fluid transfer into the periciliary layer. ,
Dysfunction of this mucociliary system causes severe lung disease from an early age. In patients with both cystic fibrosis, an inherited defect of CFTR which adversely affects the regulation of airway lining fluid homeostasis (Chapter 28), and the rare inherited condition primary ciliary dyskinesia, lung disease occurs soon after birth in many cases.
The airway lining fluid acts as a heat and moisture exchanger to humidify and warm inspired gas. During inspiration, relatively cool, dry air causes evaporation of surface water and cooling of the airway lining, then on expiration moisture condenses on the surface of the mucus and warming occurs. Thus only about one-half of the heat and moisture needed to condition (fully warm and saturate) each breath is lost to the atmosphere. With quiet nasal breathing, air is conditioned before reaching the trachea, but as ventilation increases smaller airways are recruited until, at minute volumes of over 50 L.min −1 , airways of 1 mm in diameter are involved in humidification.
Where in the respiratory tract inhaled particles are deposited depends on both their size and the breathing pattern during inhalation. Three mechanisms cause deposition:
Inertial impaction occurs with large particles (>3 µm). Particles greater than 8 µm rarely reach further than the pharynx before impaction, whereas smaller particles penetrate further into the respiratory tract. Inertial impaction is greatly influenced by the velocity of the particles, so a greater inspiratory flow rate and tidal volume will increase the penetration into the lungs of large particles.
Sedimentation occurs with particles of 1 to 3 µm, and occurs in the smaller airways or alveoli where slow gas velocity allows the particles to fall out of suspension and be deposited on lung tissue. Breath holding after inhalation of particles encourages sedimentation. Particles of this size pass easily into the alveoli, and may either diffuse back out of the alveolus to be exhaled or be deposited on the alveolar walls, where they will be absorbed into the tissue or ingested by alveolar macrophages. After deposition, different dust types have variable persistence in the lung; some are rapidly cleared, and others persist within the pulmonary macrophage for many years. Differing particle types activate the macrophage to varying extents, but may stimulate cytokine release and cause lung inflammation that then proceeds to lung tissue repair, the deposition of collagen and pulmonary fibrosis.
Diffusion, caused by Brownian motion of particles, occurs with particles less than 1 µm in size. These particles should simply be inhaled and exhaled with minimal contact with the airway or alveolar walls, although modelling studies suggest some very small particles (0.1 µm) will be deposited near alveolar openings.
Aiding all these mechanisms is the high humidity within the respiratory tract. Absorption of water by the particle during its journey along the airways will increase the particle’s mass, and so encourage both inertial impaction and sedimentation to occur. Naturally this affects hygroscopic particles to a greater degree. Any particles which are not trapped by the airway lining fluid and deposited in the alveoli are cleared by alveolar macrophages.
As an interface with the outside environment, the lung is exposed to a great many organisms carried by the approximately 10 000 L of air breathed each day. Pulmonary defence mechanisms have evolved to protect the respiratory tract from invasion by microorganisms. They can be subdivided into direct removal of the pathogen, chemical inactivation of the invading organism and, if these fail, immune defences.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here