Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
In 1895, German physicist Wilhelm Konrad Röntgen (1845–1923) published his groundbreaking research on a newly appreciated and mysterious form of electromagnetic radiation, which he designated the “x-ray.” To his amazement, these x-rays could penetrate the body's soft tissues and reveal the bones concealed from the naked human eye. Recognized with the first Nobel Prize in physics in 1901, Röntgen launched an era of medical discovery that in little more than a century has resulted in astounding in vivo anatomic and physiologic imaging capabilities.
Although conventional radiography using exposed film viewed with a light box was long the mainstay of medical imaging, the typical armamentarium for the imaging specialist has vastly expanded in the past few decades. Computed tomography (CT) and magnetic resonance (MR) imaging have now emerged as the prime noninvasive imaging methods for exploring normal and diseased brains. Moreover, hardware and software developments have transformed these initially tedious and labor-intensive methods into ones that can be quickly performed and readily obtained in most community hospitals. These modalities typically display the bony and soft tissue anatomy in slice format, but can also reconstruct digital data into stunning three-dimensional (3-D) displays. Advanced cross-sectional techniques are moving beyond mere anatomic display and are now exploring functional aspects of the brain. Functional MR imaging is able to record brain activation as patients perform simple tasks, and MR spectroscopy can analyze brain metabolites. Additionally, injectable radioisotope pharmaceuticals are revealing nuances of brain metabolism in the technique known as positron emission tomography (PET). Molecular imaging uses biomarker probes coupled with imaging tools, such as PET, to explore various molecular pathways in the brain implicated in preclinical and disease states. Optical imaging, still in its infancy, uses the absorption and scattering of visible or infrared light to analyze the chemical composition and physiologic processes of the brain. Despite advances in digital cross-sectional and functional imaging, older techniques remain essential. For example, invasive catheter angiography portrays the vascular anatomy in great detail and remains a prime method of nonsurgical treatment of vascular conditions, such as aneurysms. Interestingly, both noninvasive CT and MR vascular imaging have advanced sufficiently to replace catheter angiography in many diagnostic applications. All of these methods complement and supplement the traditional physical examination and have become essential steps in the evaluation of neurologic disease. As the frontier of neuroimaging approaches the tissue and cellular level, the neuroradiologist and neuropathologist are finding much common ground in charting a mutually rewarding partnership.
Conventional radiography was the primary method of examination for many decades until the advent of computer-assisted cross-sectional imaging in the 1970s. In conventional radiography, a penetrating x-ray beam traverses a body part. The energy of the beam is differentially absorbed depending upon the density of the tissue through which it passes. The exiting beam then exposes a film or electronic detector. The differential absorption of the x-ray beam is known as attenuation. The different attenuations account for the varied shades of gray on the exposed film or, more recently, the display monitor. The gray scale corresponds to the tissues imaged. For example, bone has a high attenuation and typically appears white or light gray, whereas air has virtually no attenuation and appears black. Conventional radiography best reveals bony anatomy and is still used in screening trauma patients. Because of the relatively minimal differences in attenuations of the intracranial tissues, conventional radiography has limited application in neuroradiology. Calcified lesions may appear dense in conventional radiography; however, noncalcified brain tumors are virtually invisible on a skull film. Historically, infusions of air into the subarachnoid space, in a process known as pneumoencephalography, and radiopaque contrast material, in the technique known as myelography, have been used to increase anatomic information. Although myelography is still widely used, especially when coupled with cross-sectional imaging, pneumoencephalography has been abandoned in favor of CT and MR imaging.
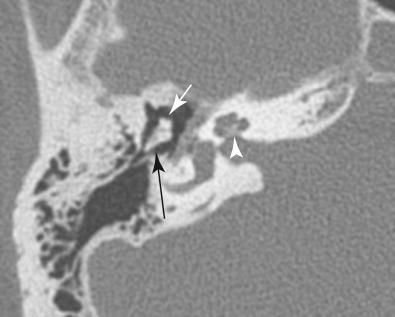
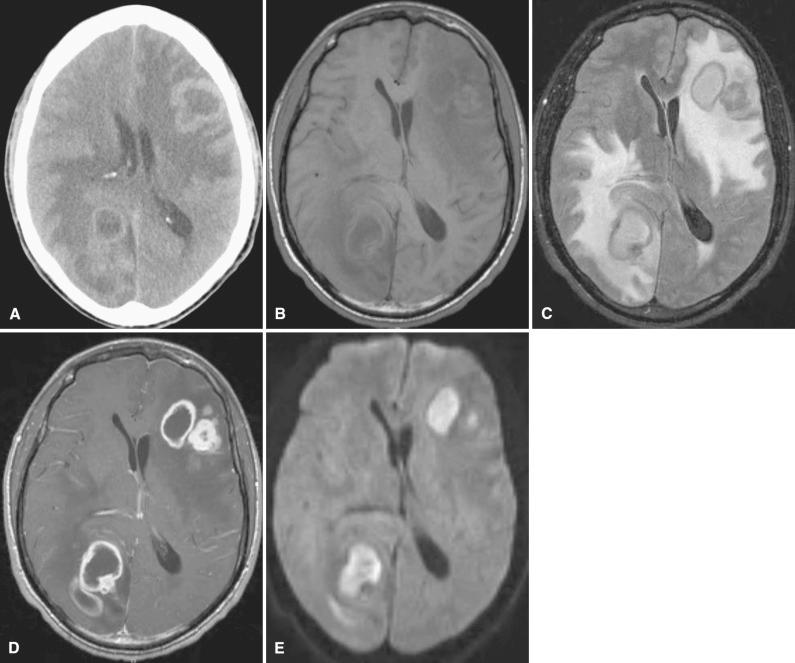
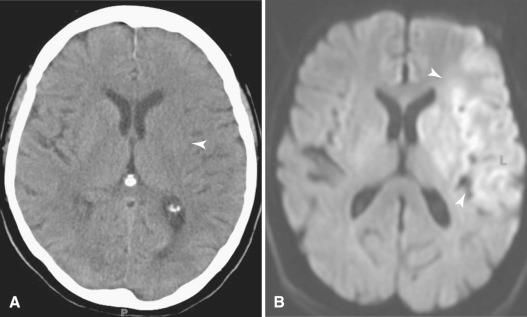
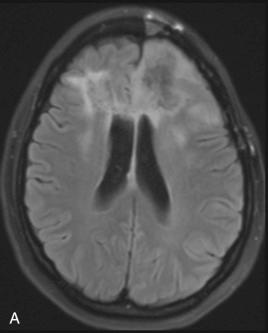
Godfrey N. Hounsfield (1919–2004) and Allan M. Cormack (1924–1998) shared the 1979 Nobel Prize in Physiology and Medicine for the development of CT, a truly revolutionizing tool that transformed neuroimaging. The method of image creation in CT relies on a beam of x-rays passing through the patient similar to conventional radiography. Rather than collecting the information in the form of film exposures as required in conventional radiography, a computer analyzes the exiting beam and reconstructs an anatomic slice in CT. CT boasts excellent spatial resolution; that is, it reliably displays small anatomic parts exceedingly well ( Fig. 4.1 ). CT not only displays bony anatomy, but also can differentiate soft tissue densities much better than conventional radiography. Therefore, CT is the technique of choice in evaluating the skull base and calvarium for subtle signs of hyperostosis, bony erosion and remodeling, or foraminal enlargement ( Fig. 4.2 ). CT also depicts cerebral tissue moderately well, especially when comparatively hyperdense material such as blood or hypodense material such as edema is present ( Figs. 4.3 and 4.4 ). Lesions containing calcium, such as meningiomas, are also fairly well detected with CT. However, CT falters when attempting to differentiate soft tissues with similar densities, such as gray matter and white matter. For example, infiltrating non-necrotic, noncalcified masses with little vasogenic edema or early infarctions with subtle cytotoxic edema may be poorly seen with CT ( Fig. 4.5 ).
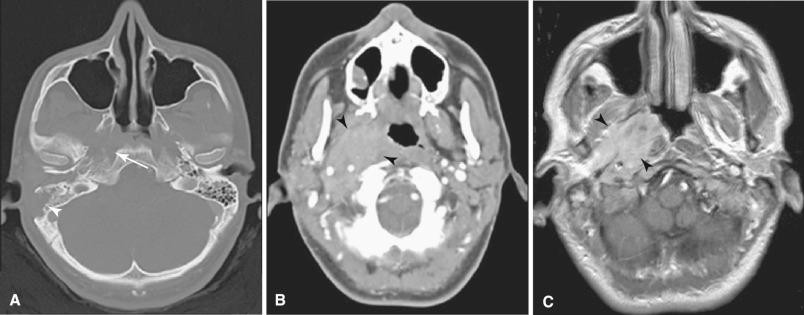
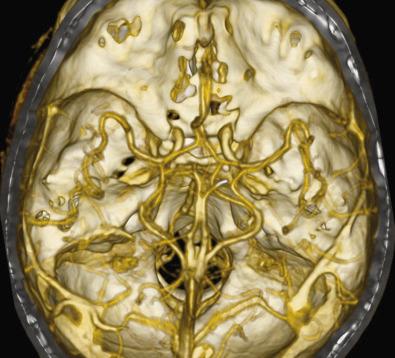
Administration of intravenous iodinated contrast material improves the visibility of cerebral vessels. Contrast material also improves the conspicuity of many brain lesions by taking advantage of the disrupted blood-brain barrier often associated with these lesions. The leakage of contrast material into the extracellular space increases the density of the lesion compared with the surrounding brain and is termed enhancement. A major advantage of CT is the rapid scanning speed. Helical or spiral technique using multirow detector technology can image the entire brain in seconds with the option of high-quality multiplanar reformations based upon the raw data. CT angiography (CTA) uses CT-acquired data to display intracranial and extracranial vessels ( Fig. 4.6 ). The resolution is inferior to catheter angiography and requires administration of iodinated contrast. Calcifications adjacent to or within vessel walls may pose difficulties in interpretation.
Dual-energy CT (DECT) is an exciting new technique that uses two x-ray spectra, most commonly with two x-ray–generating tubes running at different voltages. DECT has an advantage over single-energy CT in that it examines the varying attenuation properties inherent in different tissues. Possible clinical applications include generating virtual noncontrast images in CT angiography of the head and neck and determining the composition of arterial plaques.
CT does have shortcomings. The images produced with older machines are usually limited to axial (transverse) and sometimes coronal display. Changing the plane of section requires the patient to adjust position. Newer scanners can produce high-resolution data sufficient to allow for multiplanar reformations from the original axially oriented acquisitions. Beam hardening, a technical artifact seen especially in older scanners in which streaks obscure the anatomy, poses significant problems in the posterior fossa. Nevertheless, CT remains a valuable method in neuroradiology.
Paul C. Lauterbur (1929–2007) and Peter Mansfield (b. 1933) shared the 2003 Nobel Prize in Physiology and Medicine for discoveries that led to the development of MR imaging. MR exploits the properties of the atomic nucleus and generates images using an entirely different method than CT. In MR imaging, a magnetic field many times more powerful than the earth's magnetic field and measured in teslas (T) teams with precisely tuned radio waves to produce cross-sectional images. Hydrogen nuclei are usually targeted in clinical practice because of the abundance of hydrogen in body water. The hydrogen nuclei absorb applied energy in a process called excitation. The nuclei then achieve baseline equilibrium by releasing this energy in the form of radio waves that are detected by antennae called coils. This release of energy is known as relaxation. Relaxation consists of so-called T1 and T2 components. By adjusting the imaging parameters to emphasize either the T1 properties or the T2 properties of relaxation, tissues take on different gray scale values, which can be used when analyzing the images ( Fig. 4.7 ). No x-rays or ionizing radiation is used. The resulting MR images resolve subtle differences in the water content of tissues, such as the gray matter and white matter. Although MR imaging lacks some of the spatial resolution of CT, new and promising equipment and techniques, such as the introduction of powerful 3 Tesla (3T) magnets, have improved signal and continue to push the frontier forward.
As in CT, MR imaging portrays the brain's anatomy in slice format. MR imaging depicts this anatomy in multiple planes of orientation without the need for repositioning the patient. Also, many annoying artifacts commonly encountered with CT, such as streaking in the posterior fossa caused by beam hardening or poor x-ray penetration, do not occur with MR images. Therefore, MR scanning has become the technique of choice for studying the brain and has become indispensible in the evaluation of brain tumors.
The typical MR examination varies from institution to institution, but usually includes T1-weighted images, which are excellent for anatomic portrayal. T2-weighted and fluid attenuated inversion recovery (FLAIR) T2-weighted images emphasize free water changes that are observed in pathologic conditions such as tumors, infection, and edema. Water, such as cerebrospinal fluid (CSF) and edema, tend to be hypointense on T1-weighted images and hyperintense on T2-weighted images (see Fig. 4.7 ). Depending upon the medical indication, sequences may be obtained in the axial, coronal, or sagittal planes. Slice thickness and anatomic region of interest can also be adjusted.
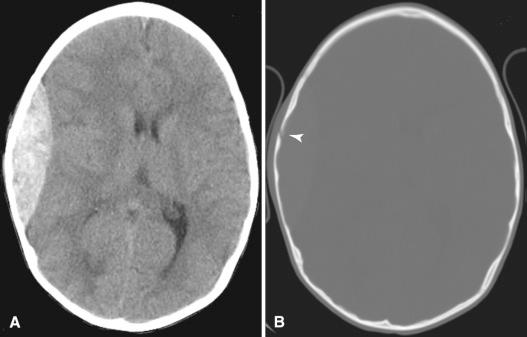
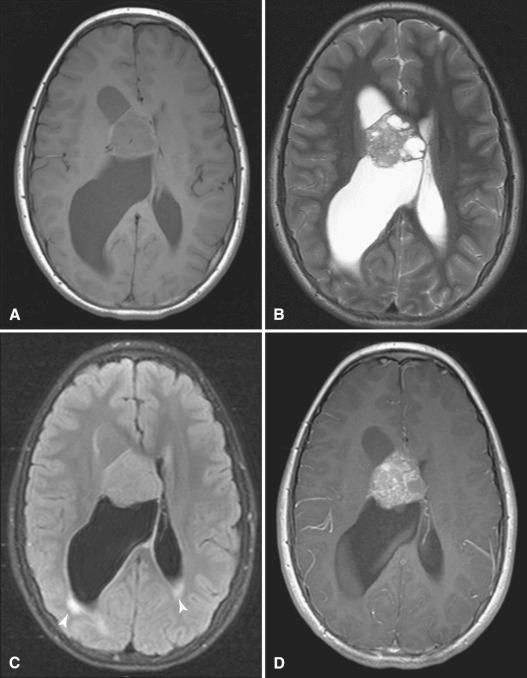
MR imaging also uses intravenous contrast to enhance detectability of lesions (see Figs. 4.4 and 4.7 ). Gadolinium-containing compounds are used with T1-weighted images, and the enhancement they provide is superior to that obtained with the iodine contrast used in CT. The use of gadolinium-based contrast agents has generally been considered safe with the exception of a rare condition known as nephrogenic systemic fibrosis/nephrogenic fibrosing dermatopathy (NSF/NFD), which has been reported in several patients with renal failure who have received gadolinium. The dissociation of gadolinium from its chelating agent is thought to cause free gadolinium deposition in vivo. In recent years, deposition of gadolinium in the basal ganglia and dentate nuclei has been reported in patients with normal renal function ( Fig. 4.8 ). The long-term implications of gadolinium deposition in the brain are currently unknown. The use of more stable agents such as gadoterate meglumine with strong ionicity and macrocyclic structures, however, is now preferred.
As in CT, MR scanning also has shortcomings. Because of the strong magnetic field, patients with ferromagnetic or electrical medical appliances such as some cerebral aneurysm clips, dorsal column stimulators, certain heart valves, cardiac pacemakers, cochlear implants, ocular metallic foreign bodies, and stapes prostheses are necessarily excluded from study because of potential appliance motion, dislodgement, and malfunction. Deaths from aneurysm clip motion within the MR device have been reported. Fortunately, medical device manufacturers cognizant of these problems and aware of the benefit of MR imaging have been designing many MR-compatible devices.
MR imaging often fails to adequately image calcifications and cortical bone; therefore, calcified lesions, fractures, and other bony pathology may be overlooked. MR scans also require more time to complete than CT, indicating patient motion may degrade the scans. Elderly, restless, anxious, or critically ill patients may have difficulty tolerating prolonged periods within the magnet when they are required to remain motionless.
Low-field so-called open MR units have become popular in recent years, but the anatomic detail of the scans produced by these machines may be insufficient to diagnose subtle lesions. Alternatively, some manufacturers are now producing short-bore 1.5-T magnets, which reduce the reported subjective sensation of claustrophobia while maintaining the superior quality of standard field strength machines.
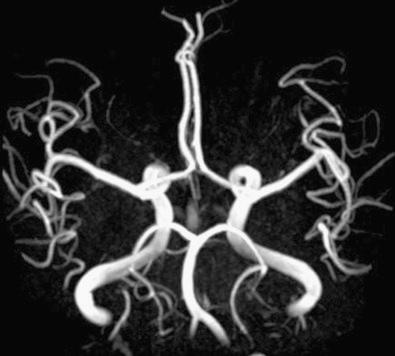
MR imaging also offers angiographic examination of the large intracranial and extracranial arteries and veins. Unlike CT angiography, MR angiography does not require the administration of contrast material, although newer protocols are successfully using gadolinium agents ( Fig. 4.9 ). Resolution is inferior to catheter angiography. Nevertheless, MR angiography may add significant screening information about the vertebrobasilar arterial system and carotid bifurcations.
Diffusion-weighted MR imaging (DWI) produces images that are sensitized to the random motion of water molecules. Processes that impede random motion of water, such as cytotoxic edema and cellular swelling in early cerebral infarction, tend to produce hyperintense regions on the scan that correspond to water diffusion restriction. Other lesions that restrict diffusion include epidermoids and cerebral abscesses. Diffusion information has also been useful in evaluating tumors. Current applications include the use of DWI in tumor grading, in analyzing peritumoral edema, in white matter tracking, and in determining postoperative injury.
The use of DWI in tumor grading is dependent on the tendency of higher-grade tumors to have increased cellularity. Cell packing increases the amount of intracellular space relative to extracellular space for a given volume of brain. The observed diffusion becomes progressively restricted with higher-grade tumors, and the signal can be analyzed on images using DWI parameters ( Fig. 4.10 ). Unfortunately, overlap of signal intensity demonstrated with tumor grades and the signal arising from tumors in which grading is not directly related to cellularity, such as atypical meningioma or lymphoma, have limited the specificity of this application of DWI. Using the rationale that hypercellularity restricts diffusion, DWI has also been used to evaluate peritumoral edema. Vasogenic edema distends the extracellular compartment with water and actually facilitates diffusion. Vasogenic edema associated with noninfiltrating tumors such as metastases and meningiomas tends to demonstrate increased diffusion, whereas infiltrating tumors such as gliomas usually restrict diffusion due to hypercellularity within the vasogenic edema. Examination of the diffusion characteristics of peritumoral edema may therefore be helpful in determining the presence of cellular infiltration.
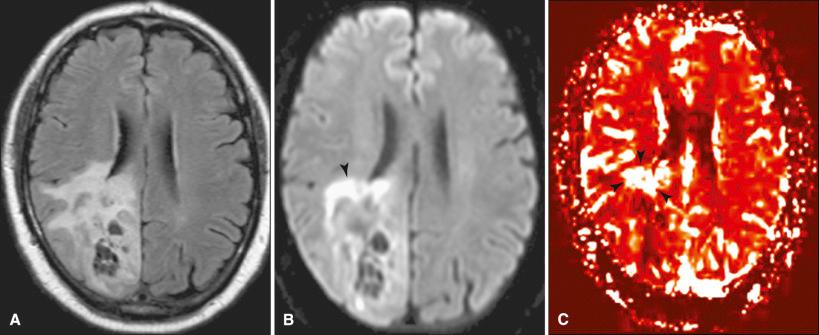
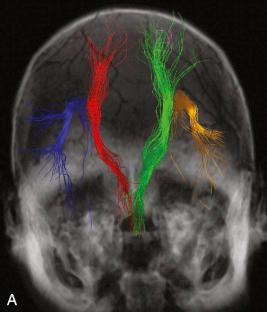
Venous infarctions or injury in the immediate postoperative period may also restrict diffusion. On follow-up scanning, enhancement may then be observed as the infarcts evolve. This enhancement may simulate recurrent tumor, but the correct diagnosis is usually suggested by referring to previous scans that demonstrate the nature of the original injury. Also, treatment with topoisomerase inhibitors and antibodies to vascular endothelial growth factor (VEGF) may reduce levels of VEGF and cause restricted diffusion within the tumor bed. This finding should not be mistaken for tumor progression. Finally, a variation of diffusion imaging known as diffusion tensor imaging (DTI) contains directional information and can be used to track white matter fibers. Tractography may reveal white matter deflection or infiltration from adjacent tumors and may be helpful in surgical planning and post-treatment evaluation ( Fig. 4.11 ).
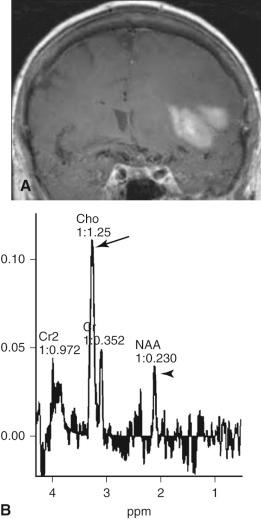
MR spectroscopy (MRS) noninvasively measures and graphically displays tissue metabolites. Ratios of various metabolites may aid in the preoperative grading of tumors. Clinical application has primarily focused on hydrogen spectra. Choline (Cho), a precursor to phosphatidylcholine, is involved in membrane turnover. A relative elevation of the Cho spectral peak within tissue implies cellular turnover and may serve as a potential tumor marker. N -acetyl-aspartate is a neuronal marker and is typically depressed in infiltrating tumors. Lactate is produced in hypoxic-driven nonoxidative glycolysis, and an elevated lactate peak may mark necrotic portions of tumors. Lipid peaks may occur in areas of membrane destruction and have been implicated in high-grade gliomas. MRS has shown promise in the metabolic mapping of tumors and may complement anatomic data from conventional imaging ( Fig. 4.12 ).
Perfusion MR imaging uses MR techniques to derive information on tumor angiogenesis and capillary permeability. One of the most intriguing parameters of perfusion imaging is the relative cerebral blood volume (rCBV) calculated for a region of interest within the brain. For example, the tumor grade in diffuse astrocytomas has correlated with rCBV, with high grades demonstrating increased rCBV, but the precise histologic explanation for the phenomenon is uncertain ( Fig. 4.13 ). Moreover, tumor grade in general cannot always be correlated with rCBV in that lower-grade oligodendrogliomas and meningiomas may demonstrate high rCBV (see Figs. 4.10 and 4.14 ). Perfusion information can also be derived from CT.
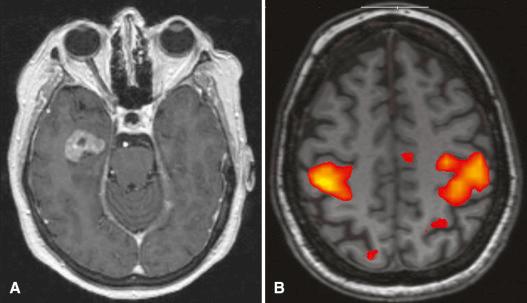
Functional MR imaging relies on the relative MR signatures of oxyhemoglobin and deoxyhemoglobin in activated cortex compared with nonactivated cortex during specific tasks such as word generation or finger tapping in order to portray areas of the brain at work ( Fig. 4.15 ). This technique has been useful in mapping the motor and language cortex prior to tumor resection. Recently, a resting state technique has been developed in which regional interactions within the brain are analyzed to derive functional information without having the patient perform specific tasks.
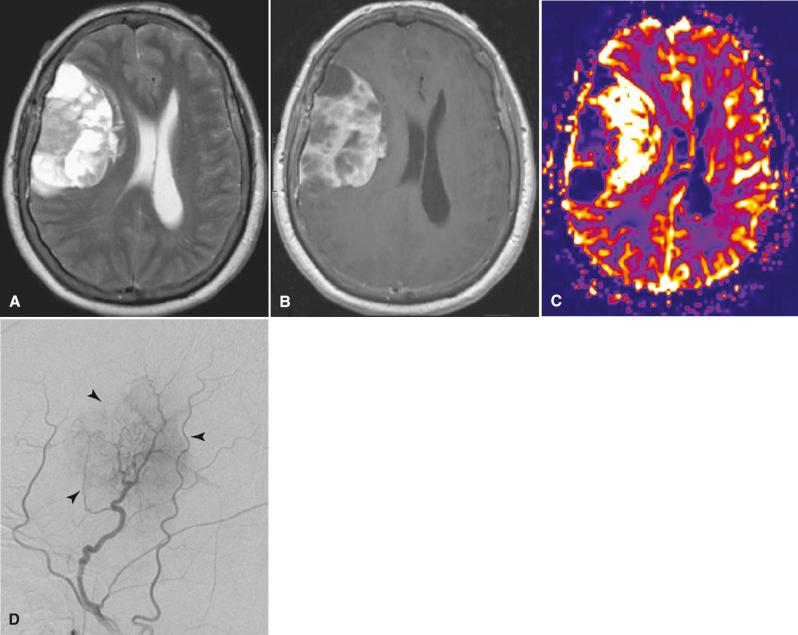
Probably one of the most mature techniques currently used by neuroradiologists is catheter angiography. Catheter angiography portrays the vascular anatomy. It is an invasive test in which a femoral artery (or in some cases, a suitable substitute such as one of the brachial arteries) is cannulated with a catheter through which iodinated contrast material is injected directly into the arteries of the neck and head. Images are made when x-rays pass through the patient and expose an image intensifier; data are then used to produce digital images. This form of angiography is considered the reference standard for all noninvasive modalities examining the blood vessels. Catheter angiography is still used to evaluate vascular patterns that may suggest a specific diagnosis, to define the feeding arteries and draining veins prior to surgery, and to plan for possible endovascular intervention ( Fig. 4.16 ). Although catheter angiography displays the blood vessels exquisitely, it does not image the surrounding soft tissues. Therefore, some of the relationships of the vessels to brain and nerves must be inferred from anatomic proximity of the vessels rather than by direct visualization of the soft tissues. With the innovative strides of noninvasive imaging, catheter angiography is now used much less frequently and is almost never used as a screening test.
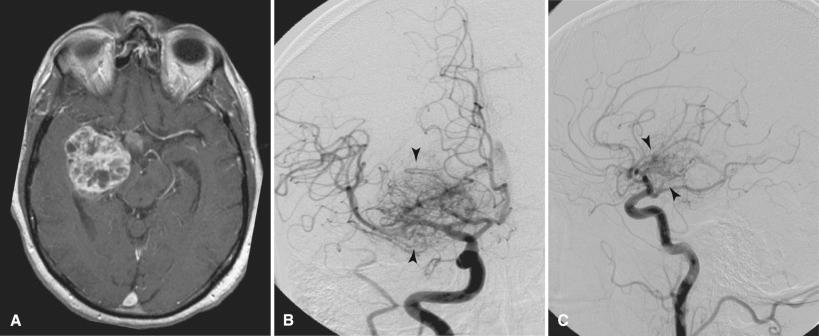
Catheter angiography can also be used as a therapeutic tool. Angioplasty, balloon occlusion, therapeutic drug infusion, the placing of arterial stents, and vascular obliteration can be performed for a variety of indications such as aneurysm and hypervascular tumors. Occasionally therapeutic angiography can circumvent surgery. Other invasive techniques include CT-guided and MR-guided biopsies. In these techniques, the superior anatomic information of cross-sectional imaging is used to guide the precise placement of the biopsy needle. CT and MR imaging may also be useful in radiation therapy planning. Conventional x-ray fluoroscopy, in which x-rays serve as a guidance tool, can also be used to direct needles and catheters for both diagnosis and therapy.
To the pathologist, gross anatomic appearances and microscopically examined histologic patterns of cellular architecture on stained tissue preparations may imply a specific tissue diagnosis. Neuroradiologists similarly rely on pattern recognition based primarily on the gray scale variations revealed in images (see Box 4.1 ). Unlike pathologists, neuroradiologists are usually confronted with the imaging slices of the entire brain. Specific signs of pathology on imaging may suggest specific diagnoses, although imaging signs (just like individual histopathology patterns) are rarely pathognomonic of a single disease process. Therefore, most neuroradiologists suggest a family of lesions that may have similar imaging appearances. Families or groups of diagnoses form differential diagnostic possibilities and are often referred to as gamuts. For example, a rim-enhancing mass may suggest an abscess, glioblastoma, metastasis, or even tumefactive demyelination. Although these diagnoses may be quite distinct clinically and pathologically, they nevertheless may appear virtually identical on cross-sectional imaging. Subtle neuroimaging clues may further refine a gamut. For example, the appreciation of a thinned margin of enhancement adjacent to the ventricular wall may suggest an abscess rather than a tumor. In addition to the imaging appearance of a lesion, a gamut may be further characterized by clinical information such as the age of the patient at presentation and the location of the lesion. For example, a rim-enhancing mass in an elderly patient with a clinical decline measured in months suggests a primary glial tumor rather than abscess or tumefactive demyelination. Although the neuroradiologist is confronted daily with numerous and often complex imaging patterns, and an exhaustive discussion is beyond the scope of this chapter, several recurrent basic patterns are useful to review.
(location, age of patient, and special features such as calcification useful in further refining differential diagnosis)
Primary glial neoplasm
Astrocytoma
Oligodendroglioma
Ependymoma
Subependymoma
Choroid plexus papilloma
Primary nonglial or mixed neoplasm
Ganglioglioma
Dysembryoplastic neuroepithelial tumor (DNET)
Central neurocytoma
Primitive/embryonal tumor (e.g., AT/RT)
Hemangioblastoma
CNS lymphoma
Metastatic disease
Tumefactive demyelination
Cerebral abscess
Intracerebral hematoma
Acute infarction
Meningioma
Hemangiopericytoma
Solitary fibrous tumor
Hemangioblastoma
Sarcomas
Schwannoma
Metastasis
Melanoma/melanocytoma
Secondary lymphoma/leukemia/plasmacytoma
Paraganglioma
Pituitary adenoma
Sarcoidosis/granulomatous diseases
Inflammatory pseudotumors
Calcifying pseudotumor of neuraxis
Primary bone tumor
Histiocytosis (e.g., Rosai-Dorfman disease)
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here