Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The World Health Organization (WHO) classification of tumors of the brain remains the worldwide standard. Members of the International Society of Neuropathology, International Academy of Pathology, and Preuss Foundation for Brain Tumor Research met in Lyon France for several bottles of wine and a lengthy discussion of whether to use “tumor” or “tumour” among other topics. Late at night, over brie and baguettes they subsequently produced the most widely used classification of brain tumo(u)rs, one that we will share with you in this book. Vive la France!
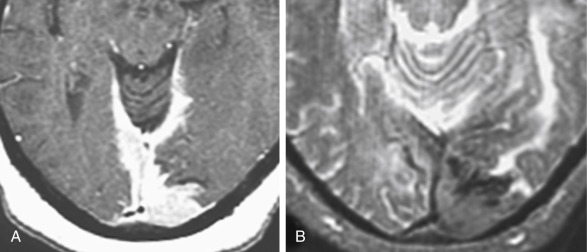
One of the essential distinctions that a neuroradiologist must make in describing a lesion is whether it is intraaxial (intraparenchymal) or extraaxial (outside the brain substance; i.e., meningeal, dural, subarachnoid, epidural, or intraventricular). This distinction has been made easier by the multiplanar capabilities of magnetic resonance imaging (MRI) and three-dimensional computed tomography (3D-CT). The quintessential and most common extraaxial dural mass is the meningioma, a readily diagnosable and treatable lesion. Extraaxial lesions buckle the white matter, expand the ipsilateral subarachnoid space, and sometimes cause reactive bony changes. On MRI scans you can visualize the dural margin with the cerebrospinal fluid (CSF cleft sign) and determine that the lesion is extraaxial. The prototypical epidural extraaxial mass, a bone metastasis, displaces the dura inward (superficial to the dural coverings), narrows the ipsilateral subarachnoid space, but otherwise may have a similar contour as an intradural extraaxial mass ( Fig. 2-1, A and B ).
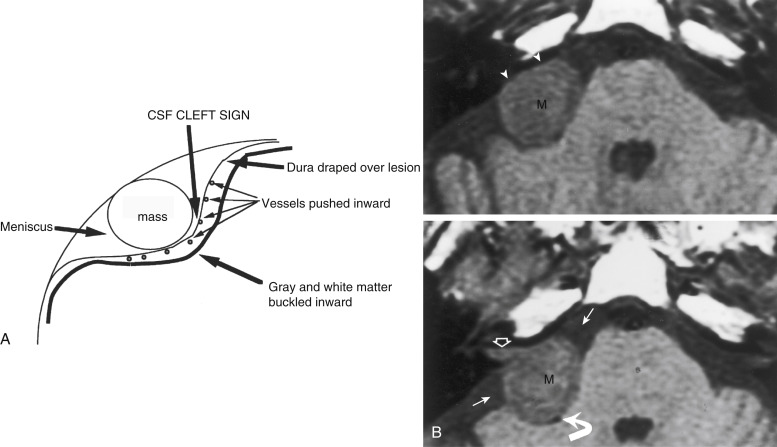
When you are confronted with a solitary intraaxial mass in an adult, the odds are nearly even that the lesion is either a solitary metastasis or a primary brain tumor (and most of these are malignant, so as the fireman says, “You’re hosed!”). Fifty percent of metastases to the brain are solitary, so the lack of multiplicity should not dissuade you from considering a metastasis in the setting of a single intraaxial lesion. You can classify a lesion as intraaxial if (1) it expands the cortex of the brain, (2) there is no expansion of the subarachnoid space, (3) the lesion spreads across well-defined boundaries, and (4) the hypointense dura and pial blood vessels are peripheral to the mass.
Occasionally, the distinction between intraaxial and extraaxial lesions may be blurred, because some extraaxial lesions may aggressively invade the underlying brain (aggressive meningiomas, hemangiopericytomas, dural metastases). Conversely, an intraaxial lesion may rarely invade the meninges (glioblastoma, lymphoma, pleomorphic xanthoastrocytomas). Nonetheless, young Jedi, you must localize a lesion as intraaxial or extraaxial so your differential diagnosis will be relevant. Once you have made that decision, you must appreciate its other qualities; its shape (margination), its internal architecture (solid, hemorrhagic, calcified, fatty, cystic), its diffusivity, and its enhancement. These are the secondary features in intraaxial and extraaxial lesions that allow you to arrive at the specific diagnosis. If you get the right diagnosis you are a Jedi neuroradiologist worthy of the “MASTER REQUISITE” distinction!
Meningiomas constitute the most common extraaxial neoplasms of the brain ( Box 2-1 ). This lesion commonly affects middle-aged women. However, because of its incidence, it represents a significant proportion of the extraaxial neoplasms in men, and in adults of all age groups. The most common locations for meningioma (in descending order) are the parasagittal dura, convexities, sphenoid wing, cerebellopontine angle cistern, olfactory groove, and planum sphenoidale. Ninety percent occur supratentorially. Because meningiomas arise from arachnoid cap cells, they can occur anywhere an arachnoid exists.
Meningiothelial, fibrous (fibroblastic), transitional (mixed), psammomatous, angiomatous, microcystic, secretory meningioma, lymphoplasmacyte-rich, metaplastic
Chordoid meningioma, clear cell meningioma, atypical meningioma, hemangiopericytoma
Papillary, rhabdoid, anaplastic
Anaplastic hemangiopericytoma (grade 3)
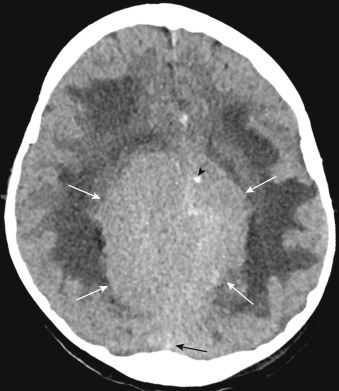
On unenhanced CT scans, approximately 60% of meningiomas are slightly hyperdense compared with normal brain tissue ( Fig. 2-2 ). You may see calcification within meningiomas in approximately 20% of cases. Rarely, cystic, osteoblastic, chondromatous, or fatty degeneration of meningiomas occurs. The specific histologic subtypes of meningioma (e.g., transitional, fibroblastic, and syncytial) cannot be readily distinguished on imaging.
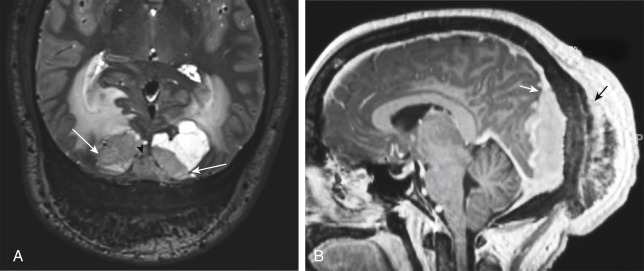
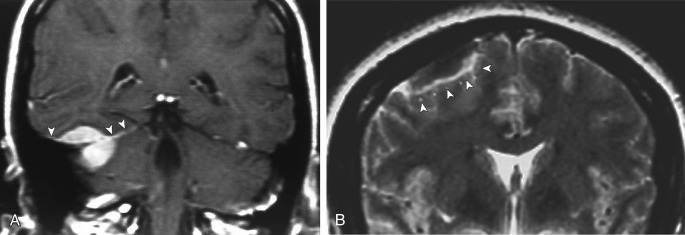
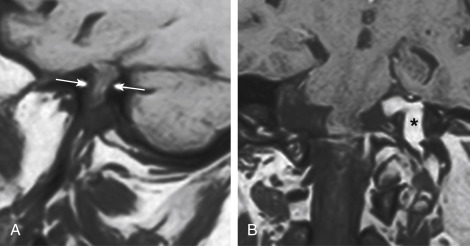
MRI is superior to CT in detecting the full extent of meningiomas, and the possibility of concomitant sinus invasion and/or thrombosis, hypervascularity, intracranial edema, and intraosseous extension. Parasagittal meningiomas may grow into the sagittal sinus and/or through the bone ( Fig. 2-3 ). Classically meningiomas are said to be isointense to gray matter on all MRI pulse sequences, but frankly they can be all over the map, given the internal architecture variability. The “cleft sign” has been described in MRI to identify extraaxial intradural lesions such as meningiomas. The cleft usually contains one or more of the following: (1) CSF between the lesion and the underlying brain parenchyma ( Fig. 2-4 ), (2) hypointense dura (made of fibrous tissue), and (3) marginal blood vessels trapped between the lesion and the brain. One may see vascular flow voids on MRI within and around a meningioma. Avid enhancement after contrast is usually seen, but occasionally, meningiomas may have necrotic centers or calcified portions, which may not enhance. With MRI you may be able to identify the “dural tail”; enhancement of the dura trailing off away from the lesion in crescentic fashion (see Fig. 2-3, B ), which is typical of meningiomas and has been exhibited in up to 72% of cases (see Fig. 2-4 ). There has been a debate in the radiologic literature as to whether the dural tail always represents neoplastic infiltration of the meninges or, alternatively, a reactive fibrovascular proliferation of the underlying meninges. In the typical situation where a differential diagnosis of vestibular schwannoma, meningioma, or fifth nerve schwannoma is debated, the dural tail may be a particularly useful sign to suggest meningioma rather than other diagnoses ( Table 2-1 ). Note, Young Skywalker, that dural metastases may demonstrate a similar finding.
| Feature | Meningioma | Schwannoma |
|---|---|---|
| Dural tail | Frequent | Extremely rare |
| Bony reaction | Osteolysis or hyperostosis | Rare |
| Angle made with dura | Obtuse | Acute |
| Calcification | 20% | Rare |
| Cyst/necrosis formation | Rare | Up to 10% |
| Enhancement | Uniform | Inhomogeneous in 1/3 |
| Extension into IAC | Rare | 80% |
| MRS | Alanine | Taurine, GABA |
| Precontrast CT attenuation | Hyperdense | Isodense |
Meningiomas may encase and narrow adjacent vessels. This finding helps in the sella region, because pituitary adenomas, the other culprit in this location, usually does not narrow the cavernous carotid artery.
Although the vast majority of meningiomas do not elicit edema, the larger ones and convexity meningiomas may cause high signal intraparenchymally even without invasion (see Fig. 2-3 ). This may be secondary to compressive ischemia, venous stasis, aggressive growth, or parasitization of pial vessels. Venous sinus occlusion or venous thrombosis can also cause intraparenchymal edema from a meningioma.
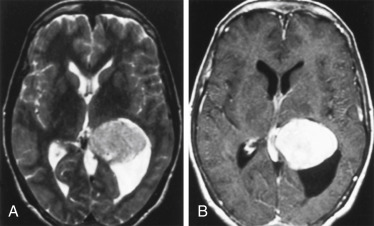
Intraosseous meningiomas may appear as expansions of the inner and outer tables of the calvarium or may even extend into the scalp soft tissues. No dural component may be present at all. This type of meningioma strongly resembles a blastic osseous metastasis and is most commonly found along the sphenoid wing ( Fig. 2-5 ). Intraventricular meningiomas typically occur around the choroid plexus (80%) in the trigone of the lateral ventricle and have a distinct propensity for the left lateral ventricle ( Fig. 2-6 ). Only 15% of intraventricular meningiomas occur in the third ventricle, and 5% occur in the fourth ventricle. Intraventricular meningiomas calcify in 45% to 68% of cases, and their frequency is higher in children. Multiple meningiomas are associated with neurofibromatosis type 2.
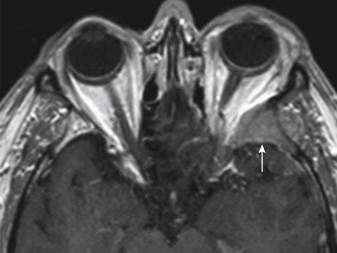
Bony changes associated with meningiomas may be hyperostotic or osteolytic, and occur in 20% to 46% of cases. Hyperostosis is particularly common when the tumor is at the skull base or anterior cranial fossa, and here it may resemble fibrous dysplasia or Paget disease (see Fig. 2-5 ). The presence of bony reaction may be a helpful feature to distinguish meningiomas from other extraaxial masses, particularly schwannomas, which do not elicit a bony reaction. If the hyperostosis is along the inner table only, you cannot say whether it is because of neoplastic invasion or reactive changes. If the outer table is transgressed, tumor is most likely. Pneumosinus dilatans is the expansion of the paranasal sinus from adjacent meningioma (usually anterior cranial fossa meningiomas and large frontal sinuses).
Meningiomas are one of the few tumors where angiography may still play an important role. Meningiomas diagnostically appear as lesions with an angiographic stain (tumor blush) and have both dural and pial blood supply. The characteristics of the stain are classically compared with an unwanted visit from your mother-in-law or Darth Maul—comes early and stays late. Depending on the location of the tumor, you may have to perform internal carotid, external carotid, and vertebral injections. In the typical convexity or sphenoid wing meningioma, the middle meningeal artery is enlarged. At present preoperative embolization of meningiomas is sometimes performed to decrease the vascularity of the tumor.
On magnetic resonance spectroscopy (MRS), meningiomas are characterized by high levels of alanine and absent N-acetyl aspartate. No glutamine is seen. One can use the presence of taurine and/or gamma-aminobutyric acid (GABA) in schwannomas to distinguish meningiomas from schwannomas.
Atypical meningiomas are classified as WHO grade II. Histopathologically they have increased mitotic rates, small cells with high nucleus/cytoplasm ratio, prominent nucleoli, sheet-like growth, or foci of necrosis. Accordingly, 2.4% of meningiomas are thereby classified as atypical and these tumors have lower apparent diffusion coefficient (ADC) values on diffusion-weighted images than typical meningiomas. They recur more frequently.
Malignant meningiomas (WHO grade III) occur uncommonly and are usually diagnosed when a meningioma exhibits intraparenchymal invasion or markedly rapid growth. Histopathologically they are defined by malignant cytology, very high mitotic rate, anaplasia, or sarcomatous degeneration. They too have restricted diffusion compared with benign meningiomas. They most likely arise from benign tumors gone awry and may aggressively invade the brain. The higher grade is associated with a higher rate of recurrence. Survival varies with the site, size, grade, and extent of surgical removal of the tumor.
Radiation therapy induces five times more meningiomas than it does gliomas or sarcomas. The diagnosis of radiation-induced meningioma is made if (1) the meningioma arises in the radiation field, (2) it appears after a latency period (of years), (3) it was not the primary tumor irradiated, and (4) it is not seen in a patient with neurofibromatosis. Multiple meningiomas occur in up to 30% of previously irradiated patients with meningiomas and in 1% to 2% of nonirradiated patients with meningiomas. Recurrence rates are higher in radiation-induced meningiomas than in non–radiation-induced tumors.
The differential diagnosis of primary tumors that mimic meningiomas is broad ( Box 2-2 ).
Lipoma
Angiolipoma
Hibernoma
Liposarcoma
Solitary fibrous tumor
Fibrosarcoma
Malignant fibrous histiocytoma
Leiomyoma
Leiomyosarcoma
Rhabdomyoma
Rhabdomyosarcoma
Chondroma
Chondrosarcoma
Osteoma
Osteosarcoma
Osteochondroma
Hemangioma
Epithelioid hemangioendothelioma
Hemangiopericytoma
Angiosarcoma
Kaposi sarcoma
Diffuse melanocytosis
Melanocytoma
Malignant melanoma
Meningeal melanocytosis
Hemangioblastoma
As noted in Box 2-2 there are a number of mesenchymal tumors that can affect the meninges. These are all relatively rare lesions, which may have either osseous (osteoma, osteosarcoma, etc.), chondroid (chondroma, chondrosarcoma, etc.), muscular (leiomyoma, rhabdomyosarcoma, etc.), fatty (lipoma, liposarcoma, etc.), or fibrous (fibroma, malignant fibrous histiocytoma) matrices associated with them. If you remember that the fibrous falx can be ossified (with bone and marrow fat), you can recall these tumors more readily.
These tumors, derived from smooth muscle pericyte cells of Zimmerman around the capillaries of the meninges, are more aggressive than most meningiomas, have a higher rate of recurrence, and can metastasize. Hence some consider them malignant and they are distinct from meningiomas. They tend to be large (over 4 cm in size), lobular, extraaxial supratentorial masses. Hydrocephalus, edema, and mass effect are not uncommon with this entity. The “mushroom sign” refers to a narrow dural attachment despite parenchymal invasion (opposite of what is expected from meningiomas) in some cases. Precontrast CT shows heterogeneous, hyperdense tumors that enhance. Hyperostosis and/or calcification is rare. Hemangiopericytomas affect men more than women, unlike most MENingiomas.
Within this category one finds diffuse melanocytosis, melanocytoma, neurocutaneous melanosis, and malignant melanoma. The melanin containing cells are leftovers from neural crest origin. These diagnoses are difficult to distinguish from metastatic melanoma to the dura. The melanocytoma is the most common of the lot and is seen in adults, whereas melanocytosis is more of a pediatric disorder. Although the latter is a diffuse process, the former usually presents as a posterior fossa mass. Hyperintensity on T1-weighted images (T1WI) is the only hope for sealing this diagnosis, but varies with melanin content. Spread of melanocytosis through the Virchow Robin spaces is possible.
Malignant melanoma of the meninges may bleed or spread from the dura to the adjacent nerves, brain, or skull. Neurocutaneous melanosis shows melanocytic nevi of the skin (especially about the face), syringomyelia, and central nervous system (CNS) lipomas. Prognosis is poor as is malignant melanoma of the meninges with distant metastases.
The intracranial neurogenic tumors (schwannomas, neurofibromas), are similar in appearance. Histologically, schwannomas arise from the perineural Schwann cells. These cells may differentiate into fibroblastic or myelin-producing cells. Two types of tissue may be seen with schwannomas, Antoni A and Antoni B tissue. The Antoni A tissue consists of densely packed palisades of fibrous and neural tissue and typically has a darker signal on T2WI because of the compactness of the fibrils. Antoni B tissue is a looser, myxomatous tissue that is typically brighter on the T2WI. Note that, depending on the degree of Antoni A and B tissue within schwannomas, the signal intensity of these lesions may be brighter than or may directly simulate that of meningiomas. Other terms used for schwannomas include neurilemmomas and neurinomas, but the most accurate term is schwannoma. Malignant schwannomas may also be seen in patients with neurofibromatosis type I.
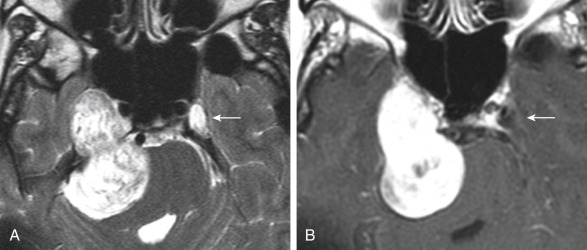
The distinction between meningiomas and schwannomas is a common one that radiologists must make at the skull base. Table 2-1 should be memorized for the top 10 ways to distinguish the two. Now OB-1! In some instances it is impossible to distinguish between the two ( Fig. 2-7 ). One of the distinguishing features of vestibular schwannomas from meningiomas is the expansion of and growth into the internal auditory canal (IAC) seen in schwannomas. The porus acusticus (the bony opening of the IAC to the cerebellopontine angle cistern) is typically flared and enlarged with vestibular schwannomas, whereas the amount of tissue seen in the IAC with meningiomas is usually small or absent. Vestibular schwannomas account for more than 90% of purely intracanalicular lesions, but only 20% of them are solely intracanalicular ( Fig. 2-8, A ). Approximately 20% of vestibular schwannomas present only in the cerebellopontine angle cistern without an IAC stem ( Box 2-3 ). About 60% of vestibular schwannomas have both a canalicular and cisternal portion (see Fig. 2-8, B ). Although it is well-known that schwannomas occur most commonly along cranial nerve VIII, the superior and inferior vestibular branches of cranial nerve VIII vie each edition of the Requisites as to which is the most common origin of the vestibular schwannoma (NOT “acoustic neuromas” and NOT the cochlear nerve). The Oscar goes to inferior division for the fourth edition. Nonetheless, despite involving the vestibular, not cochlear, nerves, the patients present with hearing loss. Classically, in “neurofibromatosis” type II, bilateral vestibular schwannomas are seen, often of different sizes.
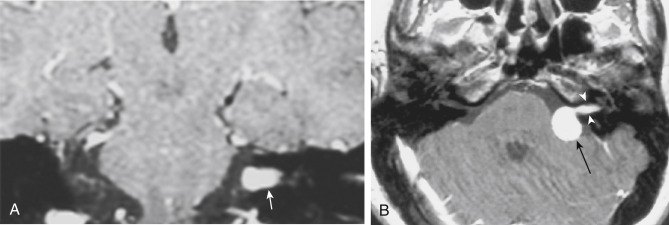
Vestibular schwannoma (75%)
Meningioma (10%)
Epidermoid (5%)
Facial nerve schwannoma (4%)
Aneurysm (vertebral, basilar, posterior inferior cerebellar artery)
Exophytic brain stem glioma
Arachnoid cyst
Paraganglioma (from temporal bone)
Hematogenous metastasis
Subarachnoid spread of tumors
Lipoma
Exophytic desmoplastic medulloblastoma
On MRI, schwannomas are usually isointense to slightly hypointense compared with white matter tissue on all pulse sequences. Enhancement is nearly always evident and is homogeneous in around 70% of cases. Peritumoral edema may be seen in one third of cases, usually in the larger schwannomas.
After lesions of cranial nerve VIII, schwannomas of cranial nerves VII ( Fig. 2-9 ) and V are the most common site of intracranial neurogenic tumors. Trigeminal schwannomas may arise anywhere along the pathway from the pons to Meckel cave ( Fig. 2-10, A and B ), to the cavernous sinus, and to and beyond the exit foramina (ovale, rotundum, and superior orbital fissure). Outside the brain, the schwannomas of cranial nerve V most commonly occur along the second division. These tumors present with facial pain that is burning in nature. Please note that ANY cranial nerve may be involved by schwannomas (except CN II, which is an extension of the CNS rather than a peripheral nerve).
Postoperatively, it is not unusual to see linear gadolinium enhancement in the internal auditory canal after vestibular schwannoma resection as a dural reaction. This can be followed expectantly. Don’t antagonize your insecure neurosurgeon by suggesting residual tumor at the outset. For those cases with progressive, nodular, or masslike enhancement, suggest recurrence.
Jugular schwannomas more commonly grow intracranially than extracranially and typically smoothly erode the jugular foramen. The border of the bone is scalloped as opposed to the paraganglioma that has a much more irregular and nonsclerotic margin. Schwannomas compress the jugular vein, whereas paragangliomas (glomus jugulare tumors) invade the vein. Jugular foramen schwannomas most commonly present with hearing loss and vertigo rather than cranial nerve IX symptoms. Whether the schwannoma arises from one cranial nerve or the next, the imaging appearance is similar.
Neurofibromas, strictly speaking, refer to the tumors associated with neurofibromatosis. They are classified as WHO grade I and all ages and sexes are represented. They may occur sporadically as well, though less commonly than schwannomas. The skin and subcutaneous tissues are affected more often than peripheral nerves; spinal nerve neurofibromas are rare and cranial nerve involvement is uncommon. These lesions contain Schwann cells, perineural cells, and fibroblastic cells and may occur in a plexiform aggressive subtype, which appears as a network of diffusely infiltrating masses. Plexiform neurofibromas in the cranial nerves or peripheral nervous system occur in neurofibromatosis type 1 (NF-1) and are one of the seven criteria for the diagnosis of this disorder. Plexiform neurofibromas, because of their extensiveness, can be distinguished from neuromas and schwannomas. They are generally found in the extremities or in the soft tissues of the head and neck. They have a significant rate of malignant dedifferentiation into a MPNST (malignant peripheral nerve sheath tumor).
Classic descriptions say that neurofibromas can sometimes be distinguished from schwannomas by their eccentricity and more fibrous central low T2 signal on MRI (“target sign”), but in many cases they two look the same.
By strict pathologic definition, neuromas refer to a posttraumatic proliferation of nerve cells rather than to a true neoplasm. The perineural lining and fibroblastic tissue seen in the other lesions just described are not present. These lesions are less common than schwannomas. They are usually seen in the cervical spine when nerves are avulsed, or in an operative bed. Again, one must know where to look for these lesions. Unfortunately, in the common vernacular most people mean “schwannoma” when they say “neuroma” (e.g., “acoustic neuroma,” which is a double misnomer as these are “vestibular” not “acoustic” [cochlear] lesions and schwannomas, not neuromas). On scanning, these masses look like small schwannomas.
Dural metastases usually spread out along the dura as hematogenously disseminated en plaque lesions from extracranial primary tumors ( Fig. 2-11 ). Lung, breast, prostate, and melanoma cancers produce most dural metastases. Some of the dural metastases may arise from spread of adjacent bone metastases. Breast carcinoma is the most common neoplasm to be associated with purely dural metastases. Lymphoma is the next most common but is unique in that the dural lymphoma may sometimes be the primary focus of the neoplasm ( Fig. 2-12 ). Dural plasmacytomas will look identical to dural lymphoma ( Fig. 2-13 ). In children, dural metastases are most commonly associated with neuroblastomas and leukemia. These tumors are also famous for lodging in the cranial sutures, widening them in an infant.
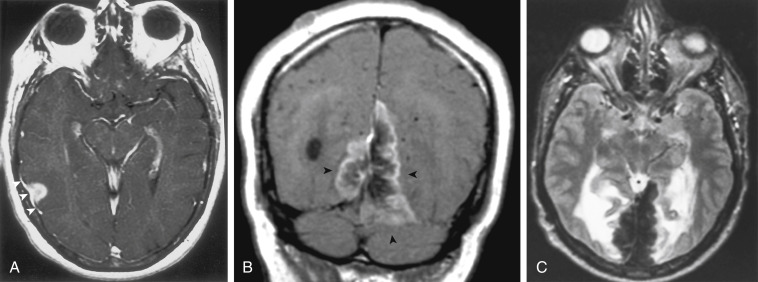
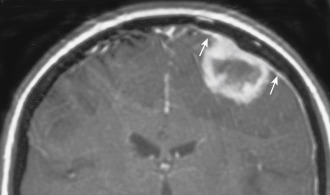
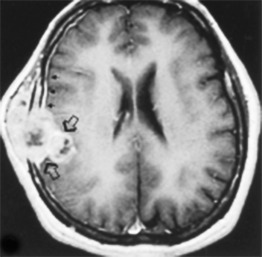
Occasionally, one can identify an adjacent parenchymal metastasis with dural spread ( Fig. 2-14 ), and, alternatively, an osseous-dural metastasis (breast, prostate primaries) occasionally invades the parenchyma ( Fig. 2-15 ). On MRI the T1WI and T2WI characteristics are variable; depending on cellular content (see Fig. 2-11, A to C ). On a CT scan these lesions are identified as isodense thickening of the meninges. Contrast enhancement is prominent. This is a diagnosis where contrast-enhanced T1WI or enhanced fluid-attenuated inversion recovery (FLAIR) scans can readily demonstrate the abnormality.
Inflammatory lesions that may simulate dural metastases include granulomatous infections (mycobacterial, syphilitic, and fungal), Erdheim Chester disease, sarcoidosis, and Langerhans cell histiocytosis.
With subarachnoid seeding (SAS) you see tiny nodules of implanted tumor on the leptomeninges. When the process is diffuse we use the term “sugar-coating” of the subarachnoid space because the whole pial surface is studded with granules. A combination of sugar coating and focal nodules (dare we say chocolate chips?) may also occur. SAS may occur with primary CNS tumors or non-CNS primary tumors ( Table 2-2 ). Lymphoma and leukemia are the most common tumors to seed the CSF. However, because they only rarely invade the meninges and do not incite reactions in the CNS, most leukemic and lymphomatous SAS is invisible on neuroimaging techniques; the diagnosis is usually made by multiple spinal taps for CSF sampling.
| CNS Primary | Non-CNS Primary |
|---|---|
| Children | |
| Medulloblastoma (PNET) | Leukemia/Lymphoma |
| Ependymoma (blastoma5PNET) | Neuroblastoma |
| Pineal region tumors | |
| Malignant astrocytomas | |
| Retinoblastoma | |
| Choroid plexus papilloma | |
| Adults | |
| Glioblastoma | Leukemia/Lymphoma |
| Primary CNS lymphoma | Breast |
| Oligodendroglioma | Lung |
| Melanoma | |
| Gastrointestinal | |
| Genitourinary | |
When a lesion has spread to the SAS, you may see it most readily on contrast-enhanced studies, and unenhanced and enhanced FLAIR imaging has been shown to be increasingly effective at identifying subarachnoid disease, including metastatic disease. The malignant cells in the CSF and/or the associated elevated protein in the CSF will cause the usually low signal of CSF to be bright on an unenhanced FLAIR scan. Although this may be a difficult diagnosis to make in the basal cisterns where “f-ing” (FLAIR and flow) artifacts abound, the presence of such high signal over the convexities implies subarachnoid seeding, subarachnoid hemorrhage, or meningeal inflammation. FLAIR with contrast enhancement increases the yield even higher!
The typical locations where one identifies SAS are at basal cisterns, in the interpeduncular cistern, at the cerebellopontine angle cistern, along the course of cranial nerves, and over the convexities ( Fig. 2-16 ). Often in non-CNS primary tumors with SAS, one identifies a peripheral intraparenchymal metastasis contiguous with the dural surface of the brain, from which cells are shed into the CSF. With subarachnoid seeding, secondary communicating hydrocephalus may be present.
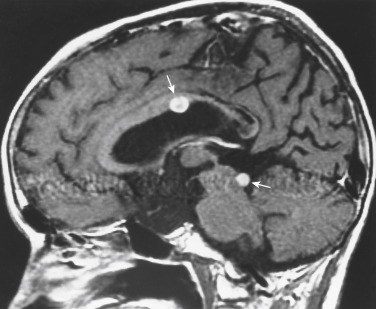
The other terms you will see for subarachnoid seeding from cancers outside the CNS include meningeal carcinomatosis or carcinomatous meningitis. Clinically, the patients present with multiple cranial neuropathies, radiculopathies, and/or mental status changes secondary to hydrocephalus or meningeal irritation. The cranial neuropathies may be irreversible. Although an initial CSF sample is positive in only 50% to 60% of cases, by performing multiple taps, the positive cytology (and post-LP headache) rate approaches 95%. Patient survival is usually less than 6 months. Breast, lung, and melanoma are the most common non-CNS primaries to seed the CSF.
Choroid plexus papillomas (WHO grade I) comprise 3% of intracranial tumors in children and 10% to 20% of those presenting in the first year of life. Eighty-six percent of these tumors are seen in patients less than 5 years old. In children, 80% occur at the trigone and/or atria of the lateral ventricles; in adults they may also be seen in the fourth ventricle. If you also include adults, 43% are located in the lateral ventricle, 39% in the fourth ventricle, 11% in the third ventricle, and 7% in the cerebellopontine angle cistern. Multiple sites are present in 3.7% of cases.
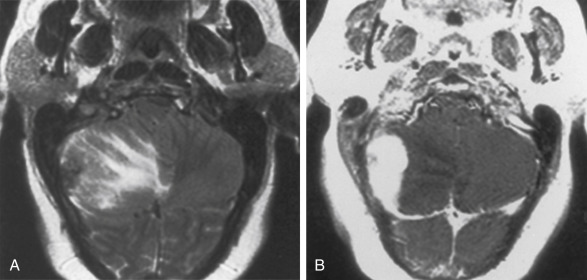
The tumors present with hydrocephalus and papilledema caused by overproduction of CSF (four to five times more than normal) or obstructive hydrocephalus caused by tumor, hemorrhage, high-protein CSF, or adhesions obstructing the ventricular outlets. Lately, the obstructionists have gained favor over the overproductionists as far as the explanation for hydrocephalus. Calcification occurs in 20% to 25% of choroid plexus papillomas, and hemorrhage in the tumor is seen even more frequently than calcification. Choroid plexus papillomas are typically hyperdense on unenhanced CT, with a mulberry appearance. They are usually of low signal on T1WI and mixed intensity on T2WI from flow voids unless hemorrhage has occurred. These tumors enhance dramatically ( Fig. 2-17 ). Between the calcification, flow voids, and/or hemorrhage, the tumor may have a very heterogeneous appearance, sometimes with a salt-and-pepper appearance from vessel supply.
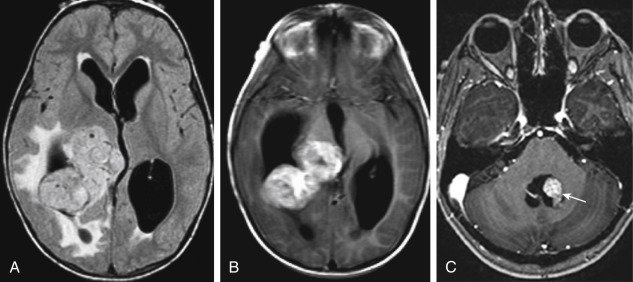
Choroid plexus carcinomas (WHO grade III) are much less common than papillomas, with malignant change occurring in fewer than 10% to 20% of papillomas. They are usually seen in the lateral ventricles. CSF dissemination is the rule with choroid plexus carcinomas, occurring in more than 60% of cases, but even benign papillomas may seed the CSF. It is difficult to distinguish a benign papilloma from a malignant one. Parenchymal invasion may suggest carcinoma. The 5-year survival for completely resected choroid plexus papillomas is 100%; for carcinomas it is more like 40%.
Choroid plexus hemangiomas are benign neoplasms of the choroid plexus usually seen in the lateral ventricle. Although the tumor enhances markedly and may calcify, it is usually seen as an incidental finding in an asymptomatic patient. There is an association with Sturge-Weber syndrome. In this syndrome choroidal hemangiomas ipsilateral to the leptomeningeal vascular malformation may be present (see Chapter 8 ).
Another benign condition of the choroid plexus is the xanthogranuloma. This incidental lesion may have fat density/intensity within it and is also centered on the glomus of the trigone. Curiously, they are frequently bright on diffusion-weighted imaging (DWI) scans ( Fig. 2-18 ). They are of no clinical import.
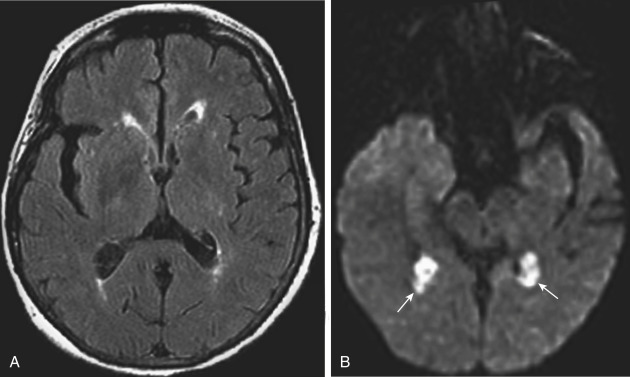
Table 2-3 lists common intraventricular neoplasms.
| Neoplasms | Lateral | Third | Fourth |
|---|---|---|---|
| Choroid plexus papilloma/carcinoma | Common, pediatric | Common, adult | |
| Craniopharyngioma | Common from suprasellar growth | ||
| Ependymoma | Common | ||
| Medulloblastoma | Common, growth from vermis | ||
| Meningioma | Common, glomus, atrium | Along choroid plexus | |
| Metastases | Yes | Yes | Yes |
| Chordoid glioma | Rare | Typical | |
| Central neurocytoma | Common, septum | Rare | Never |
Ependymomas are one of a variety of ependymal tumors ( Table 2-4 ) that may occur throughout the brain and spinal cord. Although ependymomas usually present before age 10, a second ependymoma peak in the fourth and fifth decade of life is seen.
| Tumor | Grade | Peak Age (years) |
|---|---|---|
| Ependymoma | ||
| Cellular | II | 0-9, 2nd peak, 30-50 (spinal) |
| Papillary | II | 0-9 |
| Clear cell | II | 0-9 |
| Tanycytic | II | 30-50 |
| Anaplastic ependymoma | III | 0-9 |
| Myxopapillary ependymoma | I | 30-40 |
| Subependymoma | I | 40-60 |
Posterior fossa ependymomas are usually associated with the fourth ventricle, although lesions arising primarily in the foramina of the fourth ventricle do occur and may present as masses outside the fourth ventricle ( Table 2-5 ). Although it may seem a paradox, 20% of ependymomas arise intraparenchymally, usually in the supratentorial space (thought to be because of ependymal rests left over from radial glial migration). The prognosis with ependymomas varies depending on the site; filum terminale spinal ependymomas (usually WHO grade I) do best, followed by spinal cord, followed by supratentorial space, followed by posterior fossa (usually WHO grade II) ependymomas. There is a 50% 5-year progression-free survival rate with posterior fossa ependymomas.
| Feature | Medulloblastoma | Ependymoma | Astrocytoma |
|---|---|---|---|
| Unenhanced CT | Hyperdense | Isodense | Hypodense |
| ADC Value | Low | Intermediate | High |
| Enhancement | Moderate | Minimal | Nodule enhances, cyst not |
| Calcification | Uncommon (10%-21%) | Common (40%-50%) | Uncommon (10%) |
| Origin | Vermis | Ependymal lining | Hemispheric |
| T2WI | Intermediate | Intermediate | Bright |
| Site | Midline | Midline ∗ | Eccentric |
| Subarachnoid seeding | 130% | Uncommon | Rare |
| Age (years) | 5-12 | 2-10 | 10-20 |
| Cyst formation | 10-20% | 15% | 60-80% |
| Foraminal spread | No | Yes (Luschka, Magendie) | No |
| Hemorrhage | Rare | 10% | Rare MRS |
| Metabolite | |||
| NAA | Low | Intermediate | Intermediate |
| Lactate | Absent | Often present | Often present |
| Choline | High | Less elevated | High |
Ependymomas have a greater incidence of calcification (40% to 50%) than other posterior fossa pediatric neoplasms. The calcification is typically punctate. When cysts are present (15%), they are small. On unenhanced CT scans noncalcified infratentorial ependymomas are typically hypodense to isodense. The lesions demonstrate mild enhancement, to a lesser degree than the medulloblastomas. Hydrocephalus is usually present because of blockage of the fourth ventricular outflow. They often arise as midline lesions that fill the fourth ventricle without displacing it. A classic appearance of a posterior fossa ependymoma is a calcified fourth ventricular mass that extends through and widens the foramina of Luschka and Magendie. When seen in the cerebral hemispheres, the lesions are larger and are cystic 50% of the time.
On MRI the lesions are hypointense on T1WI and tend to be intermediate in intensity on T2WI ( Fig. 2-19 ). Hemorrhage is present in about 10% of cases. Hypointensities on T2WI may be because of calcification.
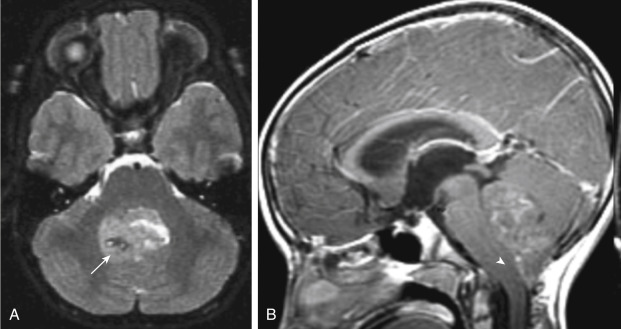
Ependymomas are another example of brain tumors that have a high incidence of subarachnoid seeding, and the use of contrast material is essential to the detection of subarachnoid spread.
Anaplastic ependymomas have an unfavorable prognosis with more rapid growth rate and more frequent contrast enhancement. The prognosis is worse with younger age, incomplete resection, subarachnoid dissemination, high cell density, and a higher rate of mitoses histopathologically. Their imaging features are indistinguishable from ependymomas.
Subependymomas are variants of ependymomas that contain subependymal neuroglia. These tumors are graded WHO grade I and often do not present until late adulthood. They arise intraventricularly or periventricularly and are frequently multiple at autopsy. They most often arise in the lateral recesses of the fourth ventricle (50% to 60%) but can be seen in the lateral ventricles (30% to 40%) attached to the septum pellucidum ( Fig. 2-20 ). Lateral ventricular subependymomas often arise in patients older than 10 to 15 years. The lesion appears isodense on CT scans, isointense on T1WI, and hyperintense on T2WI, although they may be heterogeneous lesions. Most (>60%) subependymomas of the posterior fossa do not enhance, but descriptions of lesions with minimal, moderate, and marked enhancement lead to a nonspecific characterization, particularly in the supratentorial space. They are rarely seen in the spinal canal as intramedullary or extramedullary intradural masses. Usually subependymomas are isodense to gray matter on CT and isointense on all MR pulse sequences. They have a benign course with slow growth and a lack of invasiveness. Surgical resection is curative.

This tumor may grow intraventricularly (WHO grade II tumor of neuronal origin; see Tables 2-3 and 2-6 ), and because it calcifies, simulates a much rarer intraventricular oligodendroglioma. It may be cystic, and favors the lateral or third ventricle with an attachment to the septum pellucidum ( Fig. 2-21 ). Edema is rare. Central neurocytomas peak in the third decade of life with mean age 29 years. They are isointense to gray matter on all MR pulse sequences and show mild to moderate enhancement. Intraventricular neurocytomas hemorrhage more frequently than oligodendrogliomas, which may suggest that diagnosis. Usually, however, the radiologist cannot differentiate between the two. Immunohistochemical markers for synaptophysin can distinguish intraventricular oligodendrogliomas from neurocytomas. The pathologic distinction is not moot; neurocytomas have a much more benign course than oligodendrogliomas and may not require radiation therapy. Oligodendrogliomas show a predilection for the septum pellucidum as well.

There is some confusion involved with putting epidermoids and dermoids into a “tumor” chapter because these are congenital epidermal inclusion cysts and dermal inclusion cysts. They really are not neoplastic and merely reflect two entities of ectodermal origin, one with desquamated skin (epidermoid), and one with skin appendages like hair follicles and sebaceous cysts (dermoid). Epidermoids and dermoids grow slowly and are histologically benign. Teratomas, usually lumped in the same category, however, are true neoplasms of multipotential germ cells.
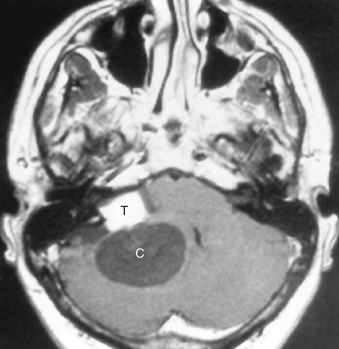
Epidermoids are also called congenital cholesteatomas and, like acquired ones in the temporal bone, have a pearly-white appearance. Men and women are affected equally, with a peak incidence in the 20- to 40-year age range. Epidermoids often occur in the cerebellopontine angle cistern (where they may present with trigeminal neuralgia and facial paralysis), the suprasellar cistern, the prepontine cistern, and the pineal region ( Fig. 2-22 ). Extradural epidermoids are nine times less common than intradural ones and arise intraosseously in the petrous bone and the temporal bone. They appear as well-defined bony lesions with scalloped margins (see Fig. 2-22, E ).
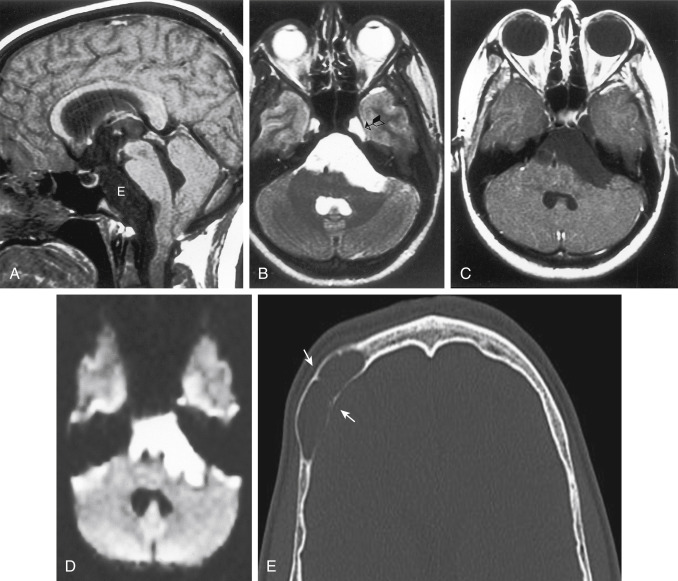
Epidermoids are typically of low density like CSF on a CT scan. Epidermoids expand to fill the interstices of the CSF space. An epidermoid is a lesion that is quite aggressive in insinuating itself around normal brain structures and often has scalloped borders. CT demonstrates a nonenhancing lobulated lesion. Sometimes epidermoids are hard to distinguish from arachnoid cysts, particularly in the cerebellopontine angle cistern ( Table 2-7 ). Classically, epidermoids do not enhance. A stereotypical appearance on a CT scan is that of displacement of the brain stem posteriorly by what appears to be just a dilated cistern anteriorly. In fact, this cistern is really a CSF-density epidermoid with mass effect.
| Characteristic | Arachnoid Cyst | Epidermoid |
|---|---|---|
| CT density | CSF | Slightly higher than CSF |
| Margins | Smooth | Scalloped |
| Calcification | No | 25% |
| Blood vessel | Displaces | Insinuates between vessels |
| Intrathecal contrast | Delayed uptake | No uptake, defines borders |
| Characteristic on MRI sequence sensitive to CSF pulsation (steady-state free precession) | Pulsates | Does not pulsate |
| Diffusion | Dark | Bright |
| ADC | Increased | Decreased |
| FLAIR | Dark | Variable |
MRI has been very helpful in distinguishing between epidermoids and arachnoid cysts, a distinction that is sometimes blurred on a CT scan. On MRI, these lesions are hypointense on T1WI and hyperintense on T2WI, similar to CSF. Most epidermoids are brighter on FLAIR than CSF, unlike arachnoid cysts. On DWI these lesions are typically very bright, showing restricted diffusion on ADC maps and thus easily distinguishable from arachnoid cysts, which are dark on DWI, and have very high diffusivity. Epidermoids do not demonstrate enhancement unless they have been previously operated or secondarily infected. Rarely, epidermoids may be bright on T1WI; this usually is because of high protein and viscosity in the lesion.
A lesion with the high intensity of fat and signal void of calcification on T1WI suggests a dermoid or teratoma ( Fig. 2-23 ). These lesions more typically occur in the midline as opposed to the epidermoids, which are generally off the midline. Male patients are more commonly affected, and patients are younger than those with epidermoids. The presence of fat may be suggested by an MRI chemical shift artifact seen as a hyperintense and hypointense rim at the borders of the lesion in the frequency-encoding direction. Fat suppression scans decrease the intensity on T1WI. The possibility of a ruptured dermoid should be considered when multiple fat particles are seen scattered on an MRI or when lipid is detected in the CSF (rule out pantopaque myelographic contrast droplets in an old, old patient). Usually the lesions are very well defined. In many cases unruptured dermoids and lipomas are not distinguishable.
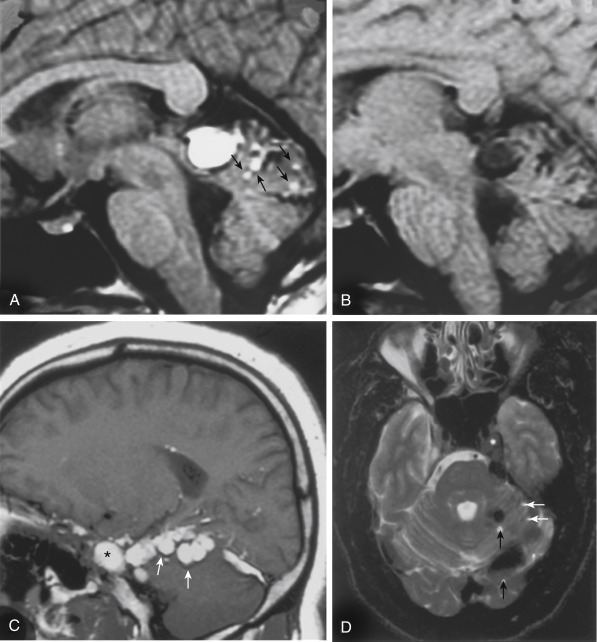
Teratomas are congenital neoplasms containing ectodermal (skin, brain), mesodermal (cartilage, bone, fat muscle), and endodermal (cysts with aerodigestive mucosa) elements.
The pineal and suprasellar regions are common sites for teratomas. One will see a lesion of mixed density and intensity. The presence of an enhancing nodule in this neoplasm may help distinguish it from a dermoid.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here