Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Natural killer (NK) cells are large, granular lymphocytes that comprise about 10% to 15% of the circulating peripheral blood lymphoctyes. These unique cells were first characterized by their ability to lyse targets independently of activating or initiating stimuli. NK cells are a critical cellular component of the innate immune system that have the ability to target and lyse malignant and virally infected cells. In addition, NK cells secrete cytokines that help to organize and shape the innate and adaptive immune response to infectious insults and malignant transformation. There is increased interest in NK cells as therapeutic agents as discoveries in the laboratory and the clinic have led to a better understanding of the important role played by NK cells in molding the early immune response. NK cells play a key role in maintaining host defense, as illustrated by human NK cell deficiency syndromes which carry increased susceptibility to overwhelming viral, intracellular, and atypical mycobacterial infections. Further, murine models of NK cell deficiency are characterized by a distinct susceptibility to development of cancer, and reduced NK cell activity in humans is associated with a higher risk for developing malignancy. This chapter provides a review of the current understanding of NK cell biology, the role of NK cells in human disease, and the recent clinical applications of NK cells to the therapy of cancer.
NK cells are critical cellular components of the innate immune response and are particularly important in the early control of viral infections and the elimination of malignant cells. NK cells express a number of receptors, both activating and inhibitory, that enable them to identify cellular targets that are not part of the host’s normal tissues. The overall balance between activating signals and the absence of inhibitory signals will lead to NK cell activation and the initiation of effector cell programs. NK cells also constitutively express a number of cytokine receptors including receptors for interleukin (IL)-2, IL-10, IL-12, IL-15, IL-18, and IL-21. The ability of NK cells to sense very low levels of these cytokines allows them to be intimately attuned to the current status of the immune microenvironment. And unlike T cells, NK cells do not require antigenic stimulation in order for cytokine receptor expression to occur. For example, resting NK cells express high levels of a functional IL-2 receptor and are able to rapidly respond to this cytokine. NK cells will embark upon the immediate cytolytic destruction of cells that do not display the appropriate “self molecules”; however, they also produce large amounts of multiple cytokines with potent effects on other immune compartments such as interferon-gamma (IFN-γ), tumor necrosis factor (TNF)-α, and granulocyte–macrophage colony-stimulating factor (GM-CSF). Notably, IFN-γ is described as the prototypical macrophage activating factor, and NK cells are known to interact with and facilitate the function of this cellular compartment as well as dendritic cells and T cells. Under the appropriate conditions, NK cells are also potent producers of chemokines such as IL-8, macrophage inflammatory protein-1-alpha (MIP-1α), and RANTES (regulated upon activation, normal T Cell expressed and presumably secreted) that have the ability to attract T cells and macrophages to sites of immunologic activity. NK cell mobilization is aided by the expression of chemokine receptors that direct their trafficking during development and differentiation and promote their recruitment to sites where an immune response is developing. NK cell adherence to the vascular endothelium is the first step in their deployment to sites of infection or malignancy and this process is facilitated via their expression of abundant levels of multiple adhesion molecules. NK cell expression of cellular adhesion molecules also promotes the formation of conjugates between NK cells and susceptible target cells. These characteristics of the NK cell compartment are indicative of a complex immune cell type with potent effector functions.
NK cells are phenotypically recognized by surface expression of CD56 (also called neural cell adhesion molecule ) and the absence of the T-cell receptor as evidenced by lack of CD3 expression. Two functional subsets of NK cells may be discriminated from one another based on the intensity of CD56 surface expression, the so-called CD56 bright and CD56 dim subsets. CD56 dim NK cells comprise 85% to 90% of the NK cells in the peripheral circulation and are generally considered to be potent mediators of cytotoxicity. About 10% to 15% of NK cells in the blood are CD56 bright , and upon activation, this subset is capable of robust cytokine and chemokine production. Fig. 21.1 graphically depicts these NK subsets, and Table 21.1 summarizes major surface antigens associated with each NK cell subset. While the use of CD56 expression is useful in understanding NK cell biology and function, it is also apparent that the differential cytolytic function and cytokine output of the bright and dim subsets is not absolute and can be altered (and even reversed) by specific environmental cues.

| Factor/Molecule a (Other Name) | Binding Partner/Function | CD56 bright | CD56 dim |
|---|---|---|---|
|
NK cell marker | ++ | + |
|
Binds Fc region of IgG | −(± in some individuals) | ++ |
|
|
+ | − |
|
Marker of differentiated NK cells with high cytotoxic/low proliferative potential | − | + |
|
|
− | + |
|
|
− | + |
| NKG2A (forms heterodimer w/ C-type lectin CD94) |
|
+ | ± |
| NKG2C (heterodimer w/ CD94) |
|
± | ± |
| NKG2D |
|
+ | + |
| NKp30 | Natural cytotoxicity receptor (NCR)Recognizes N-terminal IgV domain of B7-H6/hemagglutinin (HA) of vaccinia virus | ++ | + |
| NKp44 | NCR, expressed after NK activationRecognizes HA of influenza and Sendai viruses | ± | ± |
| NKp46 |
|
++ | + |
|
Promotes NK cell cytotoxicity and IFN-γ release upon binding to ligands poliovirus receptor (PVR, nectin-like molecule 5, CD155) or herpes entry mediator (B—HVEB, Nectin-2, CD112) | + | ++ |
|
High affinity IL-2 receptor | ++ | − |
|
Intermediate affinity IL-2 receptor | ++ | + |
| CCR5 |
|
+ | + |
| CCR7 | Receptor for CCL19, 21 (MIP-3β, 6Ckine) | ++ | − |
| CXCR1 | Receptor for CXCL1 (IL-8) | − | ++ |
|
Receptor for CXCL8 (IL-8) | + | − |
| CXCR3 |
|
++ | ± |
| CXCR4 | Receptor for CXCL12 (SDF-1α/β) | ± | ± |
| CX3CR1 |
|
− | + |
| CD44 |
|
++ | + |
| CD2 |
|
+ + | + |
|
|
++ | ± |
|
Binds to integrins CD11a/CD18, or CD11b/CD18 | ++ | ± |
|
|
+ | ++ |
| Perforin | Pore-forming cytolytic protein found in granules | − | ++ |
| Granzyme A | Serine protease induces apoptosis by cleaving/inactivating diverse substrates (e.g., mitochondrial e- transport protein) | + | ++ |
| Granzyme B | Serine protease that induces apoptosis by activation of caspases | − | ++ |
| Granzyme K | Granzyme K (GrK) and GrA have trypsin-like activity and cleave after basic residues arginine and lysine; GrB cleaves after aspartic acid residues | + | − |
a Expression levels of the listed molecules may vary across the CD56 bright and CD56 dim NK cell subsets due to donor type and variability, NK cell isolation protocols, flow cytometry gating strategies, antibody reagents being employed, and NK cell activation status.
CD56 dim NK cells are strongly cytolytic and are able to kill infected as well as tumor cell targets without prior sensitization. They constitutively express the interleukin-2/15 (IL-2/IL-15) receptor (R) β- and common γ-receptor chains, which together form a receptor complex through which cells may respond to stimulation following exposure to either IL-2 or IL-15. CD56 dim NK cells can lyse tumor cell targets through at least three distinct mechanisms. First, they can execute cytotoxicity through the exocytosis of lytic granules containing the pore-forming protein perforin and proteases called granzymes (e.g., granzyme B or GrzB) which initiate an apoptotic program via cleavage of specific protein substrates. Second, cytotoxicity can be mediated through NK cell expression of Fas ligand (CD95L) and TNF-related apoptosis-inducing ligand (TRAIL) which interact with their respective receptors CD95/Fas and TRAIL-R1 and -R2 on the target cell surface. This binding event leads to recruitment of the Fas-associated protein with death domain (FADD), recruitment and activation of procaspase 8 and 10, and the initiation of a caspase cascade that results in target cell apoptosis. This process is associated with NK cell production of cytokines, IFN-γ, TNF-α, and GM-CSF. Third, CD56 dim NK cells can mediate antibody-dependent cytotoxicity (ADCC) via the high-level surface expression of CD16 (FcγRIIIA), a low-affinity receptor for IgG which mediates a lytic program against antibody-coated target cells. Compared with CD56 bright NK cells, freshly isolated, unstimulated CD56 dim NK cells have intrinsically greater cytotoxicity in vitro against NK-sensitive tumor targets such as the well-studied K562 chronic myelogenous leukemia cell line, and they exhibit relatively high surface density expression of killer-cell immunoglobulin-like receptors (KIRs), a family of polymorphic activating and inhibitory receptors that are critically important in regulating NK cell surveillance of host tissues for cells with “altered self” (see below).
CD56 bright NK cells appear to play an immunoregulatory role in the innate immune response. CD56 bright NK produce multiple cytokines and chemokines, have a relatively high proliferative capacity, reside primarily in the parafollicular T cell–rich region of secondary lymphoid tissue (SLT), and have modest levels of cytolytic granules, low expression of KIR and FcγRIII (see Table 21.1 ). CD56 bright NK cells are unique among cytotoxic effector cells in their constitutive expression of the heterotrimeric high-affinity IL-2 Rαβγ complex, making them responsive to picomolar concentrations of IL-2 released by activated T cells in the SLT parafollicular region. As noted, CD56 bright NK cells comprise only about 10% of the circulating NK population but predominate almost to the exclusion of the CD56 dim NK subset in SLT. This distribution likely results from their selective expression of a number of receptors that assist in homing cells to SLT and retaining them there (e.g., CCR7 and CD62L). NK cell precursor expression of the C-type lectin-like surface activating receptor NKp80 correlates with NK cell functional maturity in SLTs. NKp80 may play an important regulatory role during NK maturation in SLTs related to the acquisition of cytotoxic functions and/or cytokine-production.
Chemokine receptor repertoires (and thus migration patterns) are differentially aligned among NK cell sub-populations. Compared to CD56 dim NK cells, CD56 bright NK cells express higher levels of chemokine (C-X-C motif) receptor 4 (CXCR4) which is the receptor for CXCL12 (also knowns as SDF-1α/β). CXCL12/SDF-1 stimulates NK cell migration and its production by trophoblasts is responsible for maintaining a population of CD56 bright NK cells within the decidua of the uterus during pregnancy. The CD 56 bright subset is directed to lymph nodes via their expression of CCR7, and they preferentially express CXCR3 which binds the interferon-inducible chemokines CXCL9 (MIG), CXCL10 (IP-10), and CXCL11 (I-TAC). This pattern of chemokine receptor expression, along with the high levels of the adhesion molecule L-Selectin (CD62L), helps to explain the prevalence of CD56 bright NK cells in secondary lymphoid tissues. In contrast, CD56 dim NK cells uniquely express high levels of receptors specific for inflammatory chemokines such as CXCR1, CXCR2, and CX3CR1. CXCR1 binds IL-8 (CXCL8), the levels of which are increased in the setting of acute viral infections and correlate with disease severity. Fractalkine (CX3CL1), the ligand for CX3CR1, is induced at sites of inflammation and acts as an adhesion protein for cells with CX3CR1 expression, resulting in retention of NK cells on vessel walls. As a member of the seven transmembrane domain G protein-coupled receptor family, CX3CR1 activation leads to NK cell stimulation via calcium mobilization and the initiation of mitogen-activated protein kinase (MAPK) and PI3 kinase signaling. Thus, expression of these chemokine receptors allows NK cells to traffic to local areas of inflammatory response to augment and modify the host immune response. Altered expression of chemokine ligand/receptor pairs can also promote malignant disease. In murine models of multiple myeloma, there is increased bone marrow expression of CXCL9 and CXCL10 which leads to downregulation of CXCR3 on NK cells and reduced migration and retention of NK cells to this site.
Not unexpectedly, NK cell expression of adhesion molecules also differs between the two CD56 subsets. An analysis of CD56 dim NK cells reveals that they express CD11a at a high level as compared to the bright subset. CD11a is the α chain of the αγ L β2 integrin (also known as leukocyte function associated antigen 1 [LFA-1]. Integrins bind to a wide variety of ligands in the extracellular matrix, on the surface of other cells and also soluble proteins. In contrast, CD56 bright NK cells exhibit high-level expression of CD11c. They also express high levels of CD2, CD44, CD49e, CD54, and CD62L as compared to the dim subset. CD44 binds to hyaluronic acid in the extracellular matrix. CD49e belongs to the integrin alpha chain family and associates with CD29 (integrin beta1) to form the VLA-5 receptor which binds to fibronectin and fibrinogen. CD54 is upregulated during activation and strengthens the NK-target during the lytic process. L-selectin (CD62L) is a type-I transmembrane glycoprotein that has been identified as a tethering/rolling receptor. As a result of these differing repertoires of adhesion molecules and chemokine receptors, the migratory properties of NK cells diverge quite significantly. The CD56 bright subset migrates preferentially to secondary lymphoid organs whereas CD56 dim NK cells are attracted to sites of acute inflammation.
The ability of CD56 bright NK cells to produce an abundant variety of cytokines and chemokines compared with the CD56 dim subset likely relates more to the differential expression of both negative and positive regulators of cytokine/chemokine production and less to constitutive expression of cytokine-activating receptors. For example, CD56 bright NK cells have little or no expression of two negative regulators of cytokine/chemokine production, namely SHIP-1 (Src homology 2 domain-containing inositol 5-phosphatase 1) and HLX (H2.0-like homeobox 1). CD56 dim NK cells lack constitutive expression of a positive regulator of cytokines called SET . Single-cell transcriptomic analyses generally support the use of CD56 as a means of identifying NK cell subsets with mature populations expressing high levels of CXCR1, TIM-3 (inhibitory checkpoint molecule), and ZEB2.
NK cells are prototypic, founding members of a population of cells referred to as innate lymphoid cells (ILC). Three populations of ILC have been described based on differential expression of specific transcription factors and cytokine production. NK cells are believed to arise from group 1 ILCs, which are characterized by T-bet and EOMES expression. T-bet and EOMES are T-box transcription factors that serve as master regulators of lymphoid cell differentiation. Acquisition of the IL-15 receptor is likely a first step toward NK cell differentiation from CD34 + hematopoietic stem cells and a subsequent common lymphoid precursor cell. Moreover, IL-15 is required for NK cell development in mice and humans, and the NK cell maturation process appears to occur outside the bone marrow. In humans, IL-15 has been found to regulate an NK cell signaling pathway that employs the serine-threonine kinase AKT and the transcription factor XBP1s which controls genes belonging to the unfolded protein response family. IL-15 induced accumulation of XBP1s in the NK cell nucleus enables increased T-BET-mediated transcription of the granzyme B gene. XBP1s promotes NK cell cytotoxicity and is critical for IL-15-mediated NK cell survival.
Freud et al. identified a CD34 dim CD45RA + α 4 β 7 bright cell to be the only CD34 + subset in SLT. Found within the parafollicular T cell–rich region of SLT in the same region as the CD56 bright NK cell, the CD34 dim CD45RA + α 4 β 7 bright cell can differentiate into a CD56 bright NK cell in the presence of IL-15. Five novel, discrete stages of NK cell development were characterized in situ within the same parafollicular region of SLT, each with differential expression of CD34, CD117, and CD94. As development proceeds along this continuum, the acquisition of phenotypic markers occurs in a progressive, orderly manner and cells acquire the ability to secrete cytokines (e.g., IFN-γ) and display natural cytotoxicity. This orderly development in SLT from a CD34 + subset to CD56 bright NK cells suggests that CD56 dim NK cells represent a terminally differentiated NK stage that follows CD56 bright NK development. The abundance of CD56 dim NK cells in blood versus SLT and their loss of both CD117 (c-kit) expression and proliferative capacity, along with their acquisition of KIR, FcγRIII (CD16), and cytolytic granules, are all consistent with this notion. CD57 has been identified as a surface marker of terminally differentiated NK cells.
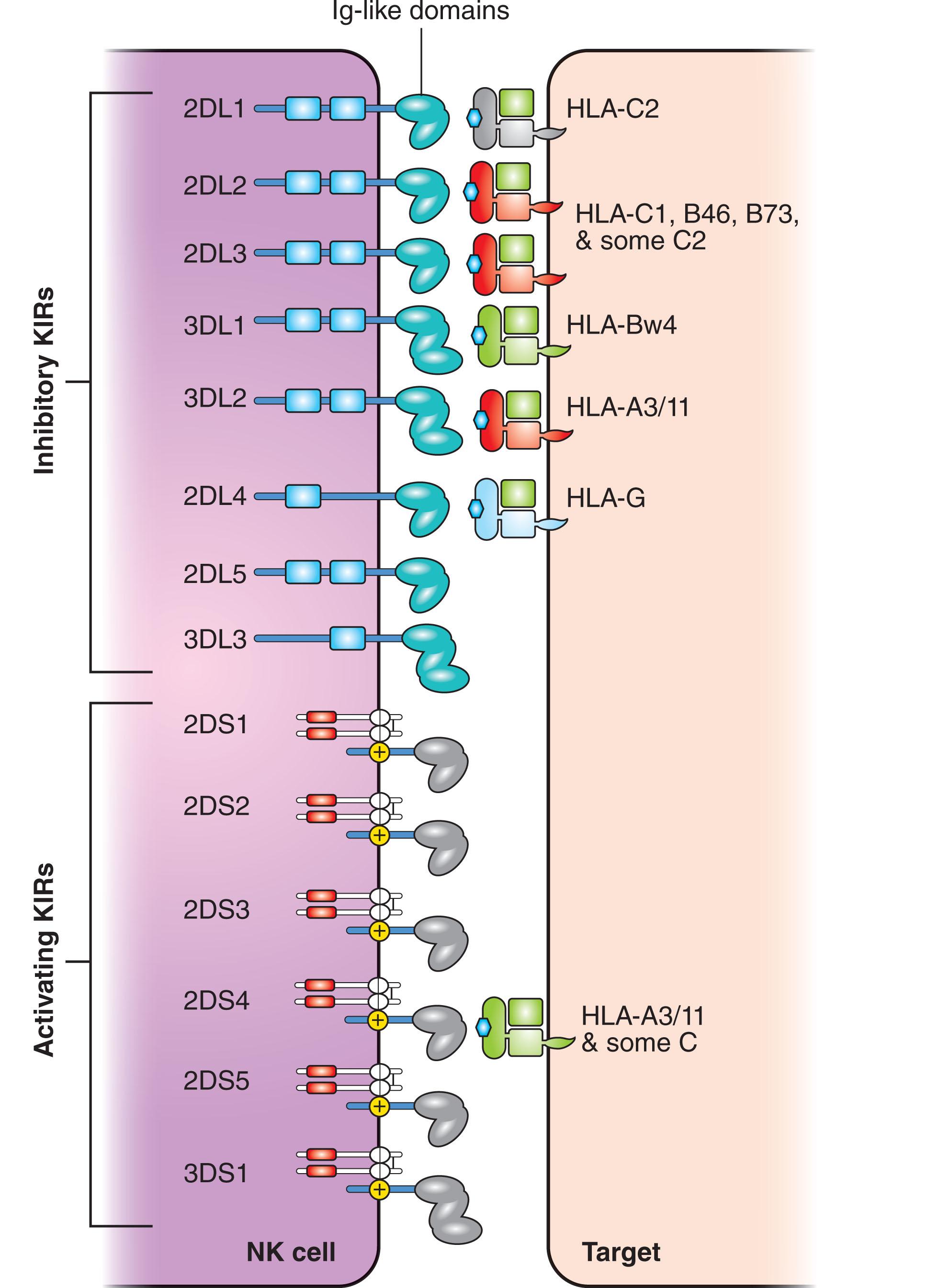
In place of antigen-specific receptors, NK cells express a complex repertoire of surface molecules that allow them to efficiently recognize malignant and virally infected cells as non-self and rapidly initiate an appropriate immune response. NK cell receptors may be activating or inhibitory. The binding of the NK cell receptor to its cognate ligand expressed on the surface of a target cell may either activate or suppress a functional NK cell response. Such receptors fall into three general categories: (1) those that are members of the KIR superfamily, (2) receptors that belong to the C-type lectin receptor (CTLR) superfamily, and (3) natural cytotoxicity receptors. Currently, investigators are working from a model in which NK cell reactivity is determined in large part by the recognition of target cell major histocompatibility complex (MHC) class I alleles, with the presence of other target cell antigens providing additional signals to the NK cell ( Fig. 21.2 ). MHC alleles are expressed on most human cells and may be classified as human leukocyte antigen A [HLA-A], HLA-B, and HLA-C and their role in suppressing NK cytotoxicity is well-established.

KIRs provide one method by which NK cells recognize self from non-self in order to mediate an appropriate cytotoxic response. In total, 16 KIR genes have been identified on chromosome 19q13.4. Structurally, the KIR protein contains two or three extracellular immunoglobulin-like domains that have the ability to recognize MHC class I proteins, namely HLA-A, HLA-B, and HLA-C. KIRs may be either inhibitory or activating, a functional feature that derives from the structure and enzymatic activity of the intracellular tyrosine-based motif of the KIR protein. An examination of KIR nomenclature provides information on the number of extracellular domains, the function of the intracellular motif, and the existence of polymorphic versions. The designation 2D or 3D indicates the number of immunoglobulin-like extracellular domains, while the length of the intracytoplasmic tail may be either long (L) and inhibitory, or short (S) and activating. A suffix numeral appearing at the very end of the KIR nomenclature indicates the existence of a polymorphic form of that particular receptor. For example, the terms KIR2DS2 and KIR2DS3 indicate the polymorphic forms of an activating KIR that displays two extracellular domains. The KIRs have been designated as members of the CD158 series.
The KIR molecules provide NK cells with the ability to sense the absence of self-HLA molecules on potential target cells—a process which was originally referred to by K. Karre and early investigators as the detection of “missing self.” The 16 KIR genes are encoded by a family of homologous genes 10-16 kb in size that have been mapped to chromosome 19q13.4 where they are tightly arranged in a head-to-tail fashion. There are 14 KIR genes and 2 KIR pseudogenes. There are eight inhibitory KIR (KIR2DL1, 2DL2, 2DL3, 2DL5, 3DL1, 3DL2, and 3DL3) and six activating KIR (KIR2DS1, 2DS2, 2DS3, 2DS4, 2DS5, and 3DS1). The pseudogenes are designated KIR2DP1 and KIR3DP1). The long tails of the inhibitory KIRs contain 1 or 2 copies of immunoreceptor tyrosine-based inhibition motifs (ITIMs) that undergo phosphorylation following interaction with specific HLA class I ligand and recruit Src homology region 2 domain containing phosphatase 1 and 2 (SHP-1, -2) that switch off the NK cell response. The short-tails of the activating KIRs lack ITIMs and instead are characterized by a positively charged amino acid residue in the transmembrane region that promotes the interaction with the 12 kDa DNAX activation protein (DAP-12). DAP12 contains immunoreceptor tyrosine-based activation motifs which initiate activating signals in response to ligand binding.
Since they are organized in the form of a tightly linked cluster, the KIR genes are inherited as haplotypes with an individual receiving maternal and paternal contributions. KIR3DL3, 3DP1, 2DL4, and 3DL2 are present in every haplotype and are referred to as “framework” genes. KIR3DL3 is located at the 5′ centromeric end of the haplotype and KIR3DL2 is located at the 3′ telomeric end with KIR3DP1 and KIR2DL4 being located in the middle of the KIR gene complex separated by a 14 kb sequence that is enriched with L1 repeats. Thus, the centromeric half of the KIR haplotype is delimited by KIR3DL3 and 3DP1, and the telomeric half is delimited by KIR2DL4 and 3DL2. The KIR genes contained within these centromeric and telomeric regions enable the KIR haplotypes to be broadly classified into two categories, namely group A or group B. Group A haplotypes exhibit a fixed gene content that consists of KIR3DL3 …2DL3…2DP1…2DL1… 3DP1 … 2DL4 …3DL1…2DS4… 3DL2 in that order, with the framework genes having been underlined. In contrast, group B haplotypes are variable both in the number and combinations of KIR genes and contain several KIR genes (2DL2, 2DL5, 2DS1, 2DS2, 2DS3, 2DS5, 3DS1) that are not part of the A haplotype. Group A haplotypes contain only one activating gene (KIR2DS4), but group B haplotypes may contain up to five different activating KIRs, namely KIR2DS1, 2DS2, 2DS3, 2DS5, and 3DS1.
KIRs recognize specific peptide motifs of HLA class I molecules which are the products of MHC genes located on chromosome 6. The human MHC encodes for six functional HLA class I genes. HLA-A, HLA-B, and HLA-C represent the so-called classical class I genes and are highly polymorphic. The genes for HLA-E, HLA-F, and HLA-G encode non-classical class I molecules that are conserved. All of these class I molecules interact with NK cell receptors with the receptors for HLA-F having only recently been identified ( Fig. 21.3 ). The alleles of the HLA-C locus can be placed into two groups of ligands (C1 and C2) based on the amino acid present at position 80 of the protein. HLA-C group 1 with asparagine at position 80 provides the ligand for KIR2DL2 and KIR2DL3, whereas HLA-C group 2 with lysine at position 80 provides the ligand for KIR2DL1. The HLA-Bw4 epitope at residues 77–83 of the HLA-B α1 domain is recognized by KIR3DL1. HLA-A3/11 serves as the ligand for KIR3DL2. While the ligands for inhibitory KIR have become well-defined, the ligand specificity of the activating KIR remains a subject of investigation. Recent studies indicate that KIR2DL4 is able to mediate both inhibitory and activating functions. Also, in addition to recognition of HLA-C1, KIR2DL2/L3 can weakly recognize some HLA-C2 alleles and the HLA-B*4601 and HLA-B*7301 alleles that bear the HLA-C1 epitope.
Genotyping efforts reveal that all human populations possess group A and group B haplotypes, but their frequencies vary considerably. People of European descent and African Americans commonly carry both A and B haplotypes, whereas the homozygous group-A KIR haplotype (AA genotype) is common in Northeast Asians (i.e., Japanese, Koreans, and Chinese). The AB or BB genotypes are common in the Indigenous populations of India, Australia, and America and the NK cells of these individuals may contain up to 6 activating KIRs. The prehistoric migrations of these populations may have provided environmental selective pressure in favor of haplotypes enriched in activating KIR.
Individual KIR genes exhibit a high level of allelic polymorphism that can affect protein expression, affinity for HLA molecules, strength of the inhibitory/activating signal, and downstream cytotoxicity and cytokine release. The inhibitory KIR exhibit greater sequence polymorphism than the activating KIRs. Generally, inhibitory receptors bind with greater avidity or attraction for a corresponding HLA antigen than activating receptors. Thus, if an NK cell expresses both activating and inhibitory KIR for an identical ligand, the NK cell will generally be inhibited from killing. Combinations of HLA variant and KIR genes exert an influence of resistance to viral infections, susceptibility to some autoimmune diseases, several pregnancy syndromes as well as the outcome of bone marrow transplantation. Specific KIR/HLA allele pairings have been associated with improved outcomes in HIV and hepatitis C infection.
KIR2DL4 is present in all KIR haplotypes, and the presence of a positively charged arginine in its transmembrane region and a single ITIM moiety enables it to generate both a weak inhibitory signal as well as an activation program that leads to the secretion of pro-inflammatory and proangiogenic cytokines. HLA-G is expressed almost exclusively in fetal trophoblast cells at the maternal-fetal interface and its soluble form is the only known ligand for KIR2DL4 which is localized to NK cell endosomes. Uptake of soluble HLA-G by NK cell endosomal KIR2DL4 leads to endosomal signaling and the secretion of a collection of factors that favors embryo implantation, placental development, and successful pregnancy.
The CTLRs, located on human chromosome 12p12.3, share a common subunit (CD94) covalently bonded to one of four closely related gene products of the NKG2 family. CTLRs promote a second type of NK cell receptor–mediated killing and include NKG2A (and splice variant B), NKG2C, NKG2E (and splice variant H), and NKG2F. NKG2D, which does not bind CD94 and shares little sequence homology to other NKG2 proteins, is discussed below. The CTLRs expressed on NK cells and cytotoxic T lymphocytes are activating except for CD94/NKG2A which is inhibitory. CD94/NKG2A specifically recognizes the non-classical HLA-E class I molecule. HLA-E*0101 and HLA-E*0103 are the only two alleles present worldwide. Tumor cells may exhibit upregulation of HLA-E expression, presumably to avoid NK cell cytotoxicity. High-level expression of HLA-E is associated with poor prognosis in several cancers, and blockade of this ligand-receptor interaction can enhance NK cell anti-tumor activity. HLA-E is able to present leader peptides from other HLA molecules which provides an additional mechanism for NK cells to sense the expression of class I MHC molecules on the cell surface. As is seen with KIR interactions, binding of CD94/NKG2A to HLA-E is more avid than binding of activating CTLRs to other epitopes.
NKG2D is a CTLR; however, it does not associate with CD94 and has limited sequence homology with other members of the NKG2 family. NKG2D exists as a homodimer and does not have intrinsic signaling capacity, but instead interacts with the DAP10 adapter molecule which is able to provide signals that recruit the p85 subunit of phosphatidylinositol 3-kinase and a complex of GRB2 and VAV1. This signal transduction arrangement effectively isolates the NKG2D signal from inhibitory signals that can suppress NK cell activation by other CTLRs. NKG2D is constitutively expressed at high levels on all NK cells, as well as on γδ T cells and CD8 + T cells. NKG2D binds to two different classes of MHC-I-like ligands whose expression is markedly induced by cellular stress, such as viral infection or malignant transformation. This binding event then leads to enhanced degranulation/cytotoxicity and production of IFN-γ. The ligands for NKG2D include the highly polymorphic MHC class I chain–related proteins MICA and MICB and 6 members of the UL16 binding proteins (ULBPs). MICA and MICB expression is under the control of promoter elements similar to those that regulate the heat shock proteins which are upregulated in malignant cells and also those infected with cytomegalovirus (CMV). UL16 is a virus-encoded type I transmembrane protein expressed in the setting of CMV infection that binds to ULBP-1 and ULBP-2 and allows the virus-infected cell to escape NK cell surveillance. Similarly some human tumors downregulate expression of NKG2D ligands or release soluble forms of MICA as a mechanism of immune escape from NK cells. Conversely, MICA expression by normal monocytes could provide a stimulus to NK cell activity against antibody-coated tumor cells.
A third family of NK receptors that participate in the process of target cell recognition and elimination mediate cell killing are referred to as natural cytotoxicity receptors (NCRs). The three major NCRs that have been found to impact NK cell activation are NKp46, NKp30, and NKp44. NKp46 and NKp30 are uniquely and constitutively expressed on NK cells, and NKp44 is expressed on NK cells after cytokine stimulation (e.g., IL-2) and also on some γδ T cells. Studies with neutralizing mAbs demonstrated that these receptors were recognizing ligands present on the surface of NK-susceptible targets, such as tumor cells and virally infected cells. The NCRs are type I transmembrane proteins that possess one or two immunoglobulin-like extracellular domains and a positively charged lysine or arginine in their hydrophobic transmembrane domain that allows them to interact with the ITAM-containing proteins and transduce an activating signal upon ligand binding. The CD56 bright and CD56 dim populations both express NKp46 and activation of this receptor promotes its association with CD3zeta or FcRgamma and drives the production of IFN-γ and TNF-α. NKp30 is expressed constitutively on all resting and activated NK cells. Its transmembrane region possesses a charged arginine residue that promotes a linkage with CD3zeta or FcRγ much like NKp46. Alternative transcript splicing gives rise to six different isoforms of NKp30, one of which (NKp30c) promotes NK cell secretion of the immunosuppressive cytokine IL-10 and is associated with reduced overall survival in patients with GIST tumors and neuroblastoma. NKp44 possesses a single extracellular V-type Ig domain that is connected to a lysine-containing transmembrane segment by a long stalk. The charged lysine residue enables the association of NKp44 with the DAP12 ITAM adapter which generates a stimulatory signal upon ligand binding. Notably, the intracellular region of the NKp44 protein contains an ITIM and thus has the ability to transmit both inhibitory and stimulatory signals. NK cell expression of NKp44 is markedly upregulated in the presence of IL-2, IL-15, or IL-1β.
A large number of NCR activating ligands have been identified which permit NK cell recognition and elimination of malignant and pathogen-infected cells. The costimulatory molecule B7-H6 is expressed at high levels on tumor cells and has been identified as an activating ligand for NKp30 that induces NKp30-dependent cytotoxicity. Some tumors shed a soluble form of B7-H6 that is associated with NK cell dysfunction and impaired tumor surveillance. Heparin sulfate (HS) glycosaminoglycans are long chains of branched anionic polysaccharides that promote tumorigenesis by altering the extracellular milieu and providing a docking site for the basic portions of secreted growth factors. Each NCR recognizes a distinct HS sequence and mediates an activating signal upon binding. NKp44 is also able to recognize nuclear proteins that undergo cell surface expression in the setting of malignant transformation or viral infection such as proliferating cell nuclear antigen (PCNA) that is normally expressed in the nucleus but can become complexed with HLA on the surface of lymphoma cells to form an inhibitory ligand for NKp44.
NKp44 and NKp46 recognize hemagglutinin-neuraminidases (HN) expressed on the surface of cells infected with parainfluenza, Newcastle and Sendai virus, and also hemagglutinins (HA) from influenza infected cells. HA from the vaccinia poxvirus also bind to NKp30 and NKp46. Virus-mediated cleavage of sialic acid residues from NKp46 disrupts its binding to HA and inhibits NK cell elimination of infected cells. NCR have the ability to recognize a broad array of pathogen-derived molecules. There is evidence that NKp44 and NKp46 play a role in the control of Mycobacterium tuberculosis and Pseudomonas aeruginosa . NKp30 and NKp46 are able to recognize proteins on the surface of erythrocytes infected with Plasmodium falciparum (cause of malaria) as well as fungal antigens from Candida glabrata , C. neoformans , and C. albicans . These beneficial effects of NCR-driven NK cell activity are balanced by studies that implicate these receptors in allergic disease and autoimmune conditions.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here