Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
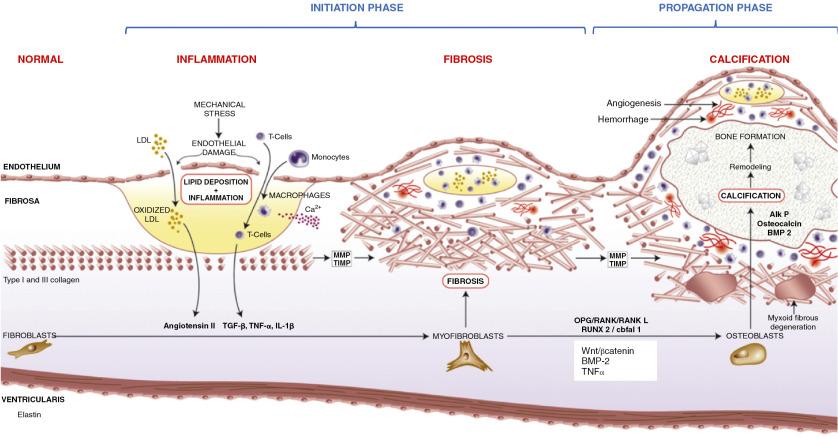
Calcific aortic valve disease is an active, highly regulated process.
The initiation phase has several similarities to atherosclerosis, including endothelial injury, inflammatory cell infiltration, and lipid oxidation.
Valve interstitial cell activation leads to pathologic extracellular matrix remodeling and valvular fibrosis.
Procalcific processes occur in the valve under the control of highly regulated osteoblast-like signaling (osteogenic) or passive calcium deposition and accumulation (dystrophic).
The propagation phase eventually supersedes with a self-perpetuating cycle of mineralization, vascular injury, and disease progression.
Sustained pressure overload leads to a pathologic hypertrophic response in the left ventricle that is characterized by myocardial fibrosis, subendocardial ischemia, and cell death, ultimately resulting in left ventricular decompensation.
Aortic valve disease represents a significant global health challenge. The prevalence of aortic stenosis increases exponentially with age and is the most common valve disease requiring surgery in the Western world. Severe disease is estimated to affect 3.4% of people older than 75 years of age.
Aortic stenosis is characterized by progressive narrowing of the valve and by the way in which the left ventricle (LV) adapts to the increased afterload imposed on it. Symptoms and adverse events are related to processes occurring in the myocardium and the valve. This chapter focuses on the molecular mechanisms underlying progressive narrowing of the valve and on the hypertrophic response of the LV. It also highlights potential therapeutic targets for this common clinical condition.
The normal aortic valve consists of three cusps attached at the base to the fibrous aortic root annulus and that coapt along their free edges during ventricular diastole to prevent retrograde flow into the LV. The cusps are usually less than 1 mm thick and consist of three layers: fibrosa, spongiosa, and ventricularis ( Fig. 3.1 ).
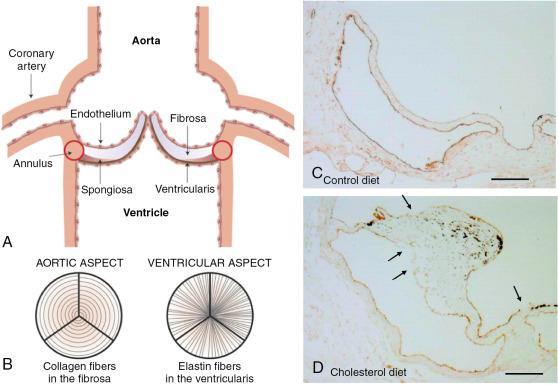
The fibrosa layer faces the aorta, consists of collagen types I and III arranged circumferentially, and acts as the main load-bearing layer. The spongiosa connects the outer fibrosa and ventricularis layers, helping to lubricate their relative motion as they deform during the cardiac cycle. This layer predominantly comprises proteoglycans with a smaller percentage of collagen fibers and fibronectin and laminin as adhesive proteins. The ventricularis layer faces the LV and contains radially orientated elastin fibers in addition to collagen. The elastin helps to mitigate radial strain when the valve is fully open and increases recoil to aid valve closure ( Table 3.1 ).
| Cell Type | Origin | Function |
| Endothelium | Endothelial endocardial cushion | Paracrine regulation of VIC function Maintenance of VIC population through ECM |
| Circulating endothelial progenitors | Repair in response to injury Maintenance of VIC population through ECM |
|
| Quiescent, resident interstitial cells | Endothelial endocardial cushion (by ECM) or neural crest | Maintenance of valve structure/connective tissue production Secretion of antiangiogenic factors Potential osteogenic precursor |
| Interstitial cells of extravalvular origin | Bone marrow/circulating progenitor cells | Repair in response to injury Potential osteogenic precursor |
| Activated interstitial cells (α-SMA + ) | Resident or circulating immune complex cells | Repair in response to injury (migration, proliferation) Angiogenic factor secretion with cusp thickening Robust ECM production/matrix remodeling enzyme expression Potential osteogenic precursor |
Calcific aortic valve disease was long dismissed as a degenerative condition in which progressive “wear and tear” resulted in passive accumulation of calcium in the valve cusps. However, emerging evidence indicates that aortic valve disease instead develops as part of a highly complex and tightly regulated series of processes, each of which is potentially amenable to medical intervention.
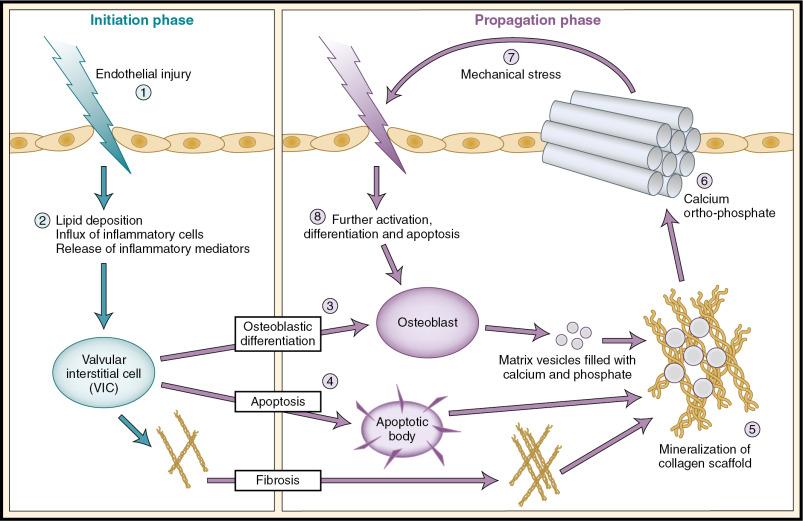
Contemporary thinking is that the pathophysiology of aortic stenosis can be subdivided into two distinct phases: an early initiation phase with many similarities to atherosclerosis, in which endothelial injury, valvular lipid deposition, and inflammation dominate, and a later propagation phase, in which osteogenic and procalcific mechanisms dominate and drive progressive valve narrowing ( Fig. 3.2 ). ,
Although the description of cellular and molecular changes in human tissue is a critical component in research, empirical testing in animal models can provide important insights about whether a change is a pathophysiologic driver of calcific aortic valve disease or merely an epiphenomenon. When evaluating experimental data or deciding which model is useful for a particular study design, several important questions must be addressed ( Table 3.2 ).
Does the model require genetically altered animals, and do the mutations relate to the specific question at hand?
What is the underlying stimulus driving calcification (e.g., hyperlipidemia, hypertension)?
Are the histopathologic changes relevant to human disease (e.g., fibrosis, calcification)?
Do the animals develop hemodynamically relevant aortic valve dysfunction and stenosis or only aortic valve sclerosis?
| Species/Strain | Diet | Histopathologic Changes in Aortic Valve | Hemodynamically Significant Stenosis |
|---|---|---|---|
| Mice | |||
| C57BL/6 | HF | Lipid deposition Modest calcification |
No |
| ApoE −/− | Chow | Lipid deposition Calcification Monocyte/inflammatory cell infiltration |
<2% |
| HF/HC | Lipid deposition Fibrosis Calcification Monocyte/inflammatory cell infiltration |
<2% | |
| Ldlr −/− | HF/HC | Lipid deposition Calcification Monocyte/inflammatory cell infiltration |
No |
| Ldlr −/− /apoB 100/100 | Chow | Lipid deposition Calcification Monocyte/inflammatory cell infiltration Myofibroblast activation |
Yes, ≈30% of mice |
| HF/HC | Lipid deposition Calcification Fibrosis Monocyte/inflammatory cell infiltration Myofibroblast activation |
Yes, >50% of mice | |
| EGFR Wa2/Wa2 | Chow | Fibrosis Calcification Inflammatory cell infiltration |
Yes, but background strain dependent |
| eNOS −/− | Chow | Bicuspid aortic valves in ≈40% of mice | No |
| Tricuspid offspring | HF/HC | Calcification Fibrosis |
No |
| Bicuspid offspring | HF/HC | Calcification Fibrosis |
Yes |
| Notch1 +/− | HF/HC | Calcification | No |
| Periostin −/− | Chow | Calcification Fibrosis |
Not known |
| HF/HC | Reduced valve thickening and fibrosis | No | |
| MGP −/− | Chow | Calcification | Not known |
| Klotho −/− | Chow | Calcification | No |
| Col1a2 Oim/Oim | Chow | Fibrosis/extracellular matrix disruption | No |
| Twist1 Tg/0 | Chow | Hypercellular, thickened valves | No |
| Sox9 Fl/+ /Col2a1 Cre | Chow | Calcification Fibrosis |
No |
| Chm1 −/− | Chow | Neoangiogenesis Lipid deposition Calcification |
Not known |
| Rabbits | |||
| New Zealand White | HF/HC | Lipid deposition Calcification Inflammatory cell infiltration |
<10% Mostly moderate sclerosis |
| Chow + hypertension | Fibrosis Inflammation |
<10% | |
| Watanabe | HF/HC | Lipid deposition Fibrosis Calcification Inflammatory cell infiltration |
No |
| Pigs | |||
| Yorkshire Landrace | HF/HC | Lipid deposition | No |
One criticism of preclinical animal models is that they have largely failed to accurately reflect human aortic stenosis, which has delayed development of effective medical therapies. Because of the difference between the human condition and that induced in animal models, data from the latter must always be interpreted with a degree of caution.
The initiating event in aortic stenosis is thought to be endothelial injury related to increased mechanical stress and reduced sheer stress. The aortic aspect of the valve cusps experience reduced shear stress compared with the ventricular aspect, with the noncoronary cusp experiencing even lower shear stress due to the absence of associated coronary blood flow. This may explain why valvular lesions appear to favor the aortic aspect of the valve and the noncoronary cusp in particular. This is illustrated using the model of bicuspid aortic valve disease. Two rather than three valve cusps result in less efficient dissipation of mechanical stress, and as a result, patients with bicuspid valve disease almost universally develop aortic stenosis with more rapid disease progression, and on average, they require aortic valve replacement 5 to 10 years earlier. ,
This early initiation phase of the disease is somewhat similar to atherosclerosis, with endothelial injury, lipid infiltration, and inflammation predominating. This may explain why the incidence of aortic stenosis is related to risk factors that are similar to those of atherosclerosis, with large longitudinal studies consistently demonstrating an association with factors such as age, smoking, hypertension, and hypercholesterolemia ( Table 3.3 ). Later in the initiation phase, angiogenesis can be observed alongside matrix remodeling and the earliest stage of valvular calcium formation.
| Risk Factor | Potential Molecular Mediators |
| Hypertension | Angiotensin II Force/shear-initiated signaling pathways Reactive oxygen species |
| Diabetes | Hyperglycemia Receptor for advanced glycation end products (RAGE) activation Angiotensin II Reactive oxygen species |
| Hyperlipidemia | Low-density lipoprotein Lipoprotein-related receptor protein 5/6 activation Local angiotensin II generation Reactive oxygen species |
| Smoking | Reactive oxygen species |
| Aging | Epigenetics Reactive oxygen species |
Similar to atherosclerosis, endothelial injury in aortic stenosis is followed by valvular lipid infiltration, predominantly lipoprotein(a) and low-density lipoprotein (LDL) cholesterol. Observational studies have shown that total cholesterol, , LDL, and lipoprotein(a) , are independent risk factors for the development of aortic stenosis; high-density lipoprotein appears to be protective.
Aortic valve calcification was linked to a single nucleotide polymorphism in the lipoprotein(a) locus in a large genome-wide linkage study. This association led to hope that statin therapy might modify the progression of aortic stenosis and was supported by preclinical data (using the hypercholesterolemic mouse model of aortic stenosis). However, three randomized, controlled trials demonstrated that statins did not modify the natural history of this disease, suggesting that although lipid deposition and inflammation are involved in disease initiation, they are less important in driving disease progression.
The pathologic processes implicated in the propagation phase of aortic stenosis appear to be different from those in early initiation, but there has been renewed interest in lipid-lowering agents to modify disease progression since the development of the proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors. PCSK9 is a hepatic convertase that internalizes LDL receptors, and PCSK9 inhibition can lead to dramatic reductions in circulating levels of LDL and lipoprotein(a). The PCSK9 R46L loss-of-function mutation was associated with a lower risk of developing aortic stenosis in a large Danish observational study, and in a small cross-sectional study, PSCK9 levels correlated with the presence but not the severity of aortic valve disease. A randomized trial (NCT03051360) comparing the effect of PCSK9 inhibitor therapy with placebo on disease progression in patients with aortic stenosis is underway.
Similar to atherosclerosis, lipid deposition and oxidation in the valve induces a proinflammatory response. It is characterized by chemotaxis and infiltration of macrophages, T lymphocytes, and mast cells. Inflammation in the valve may also be observed in the later stages of the disease related to recurrent valve injury, reactive oxygen species, cell death, and increased mechanical stress in the valve.
Consistent with this finding, elaboration of proinflammatory cytokines (e.g., tumor necrosis factor-α [TNF-α], interleukins IL-6 and IL-1) is also dramatically increased in humans and animals with calcific aortic valve disease. Although few studies have examined the role of proinflammatory cytokines in the progression of calcific aortic valve disease in humans, three lines of evidence from nonclinical work suggest that they may play a role in initiation and progression of the disease.
First, aortic valves from IL-1 receptor antagonist (IL-1ra)–deficient mice are thickened, accumulate calcium, and develop mild aortic valve dysfunction (peak transvalvular velocity 2 m/s). This phenotype is abolished in IL-1ra/TNF-α double-knockout mice, suggesting that TNF-α is the major downstream mediator of IL-1–induced inflammation at least in this murine model.
Second, a growing body of work suggests that activation of receptors for advanced glycosylation end products (RAGEs) is likely to accelerate cardiovascular calcification. Specifically, overexpression of S100A12 accelerates vascular calcification in hypercholesterolemic mice by what appears to be a nicotinamide adenine dinucleotide phosphate (NADPH) oxidase–dependent mechanism. , RAGE activation drives proinflammatory cytokine production and osteogenic gene expression in valve interstitial cells (VICs) in vitro. Although mechanisms contributing to increased oxidative stress differ dramatically between the aorta and aortic valve (discussed later), , numerous studies have shown that RAGE activation is strongly associated with increases in TNF-α, , which may be a point of convergence in inflammatory signals driving calcification in the aortic valve and aorta.
Third, addition of exogenous TNF-α to cultured aortic VICs amplifies bone morphogenetic protein (BMP) signaling and accelerates calcium accumulation in vitro. TNF-α accelerated calcification only in cells from patients with calcific aortic valve disease (i.e., not in cells from nonstenotic control valves), suggesting that phenotypic and/or epigenetic changes that occur in vivo may persist in cultured VICs in vitro. The molecular mechanisms by which TNF-α promotes VIC calcification are still being investigated, but work in aortic myofibroblasts suggests that reactive oxygen species (ROS) generated by TNF receptor 1 activation may be integral. ,
Active inflammatory processes are sustained by angiogenesis, the process by which new blood vessels form from existing capillaries. This becomes particularly important when the valve becomes grossly thickened and the central components become relatively ischemic. Angiogenesis is seen in 85% of valve leaflets in patients with aortic valve disease and co-localizes to areas of inflammation, fibrosis, and ossification.
Angiogenesis is regulated by angiogenic factors such as vascular endothelial growth factor (VEGF), and fibroblast growth factor 2 (FGF2) and by several inhibitors. The balance of proangiogenic and antiangiogenic factors is altered in calcific valve disease. For example, the antiangiogenic glycoprotein chondromodulin 1 is downregulated in the extracellular matrix (ECM) of diseased valves, which is then associated with angiogenesis. New vessels that develop in calcific aortic valve disease are leaky and prone to rupture. This can cause valve leaflet hemorrhage, a potentially important trigger for further valve calcification. ,
Substantial changes in the composition, organization, and mechanical properties of the ECM are observed in stenotic aortic valves. Progressive fibrosis leads to leaflet thickening and is characterized by increased deposition of collagen in all valve layers, along with associated remodeling and disorganization. This pattern appears to be mediated in part by increased activity of matrix metalloproteinases (MMP1, MMP2, MMP3, and MMP9), their tissue inhibitors (TIMP1 and TIMP2), , and cathepsins, which are potent elastolytic enzymes. ,
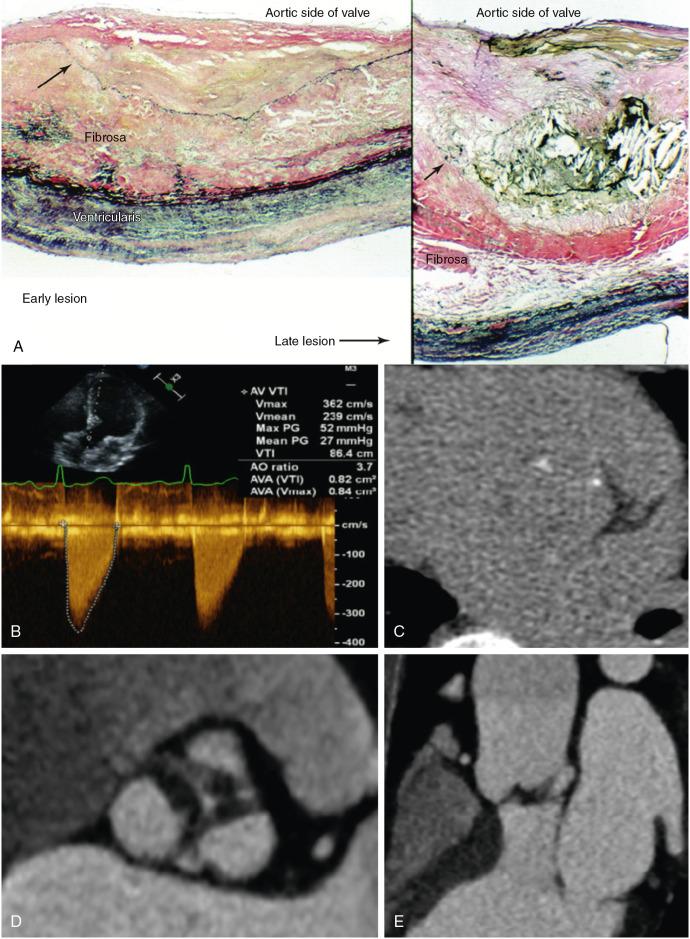
Progressive fibrosis of the valve contributes alongside calcification to increases in aortic valve stiffness, thereby directly resulting in progressive stenosis of the valve. In a small minority of often younger patients with aortic stenosis, fibrosis appears to be the predominant process responsible for valve narrowing with minimal or absent calcification , ( Fig. 3.3 ). However, much more commonly extensive valvular calcification is associated with increases in ECM accumulation and turnover. , The profibrotic processes that occur in the valve are thought to play a role similar to that observed in skeletal bone formation, providing a scaffold on which subsequent calcification occurs. Fibrosis and calcification therefore appear to be closely linked and the major drivers of aortic stenosis progression.
Activation of the renin-angiotensin system is implicated at multiple levels in the pathogenesis of aortic stenosis in the valve and in the myocardium. The renin-angiotensin system is involved in the development of hypertension and may therefore accelerate valve disease progression by associated increases in valvular mechanical stress. Angiotensin II also has proinflammatory and fibrotic effects at the local valve level, where profibrotic effects appear to be mediated by the angiotensin II type 1 (AT1) receptor. Although angiotensin II can have antiinflammatory and fibrotic effects by type 2 (AT2) receptors, they are downregulated in calcific aortic valve disease. Similarly, angiotensin-converting enzyme type 2 (ACE2), which mediates antifibrotic and antiinflammatory effects by the Ang(1-7)/MAS pathway is also downregulated. Overall, the renin-angiotensin system plays a predominantly profibrotic and proinflammatory role in stenotic aortic valves.
Angiotensin II is upregulated in two ways. First, there is increased ACE activity in calcific aortic valve disease, which is likely delivered to the valve by its natural vehicle, LDL, after endothelial injury. Second, infiltrating macrophages are abundant and can act as primary sources of increased tissue angiotensin II concentrations due to their expression of chymase, which converts angiotensin I to angiotensin II.
Although retrospective clinical studies suggest that ACE inhibition may slow progression of aortic valve disease, there is a lack of randomized, controlled trial data, and their lack of effect on chymase activity makes ACE inhibition a less appealing target. Preclinical experiments in hypercholesterolemic rabbits showed that angiotensin I receptor blockade attenuates aortic VIC activation, endothelial disruption, and valvular inflammation in early stages of valve disease, suggesting that direct angiotensin inhibition may also be useful.
VICs are similar to activated myofibroblasts and appear to be the key coordinators of valvular fibrosis and later calcification. The interactions between VICs and their environment appears important in the regulation of fibrosis and calcification, and they can be functionally categorized as matricellular signaling, matricrine signaling, mechanical signaling through changes in matrix elasticity, and mechanical signaling due to changes in external forces ( Fig. 3.4 ).
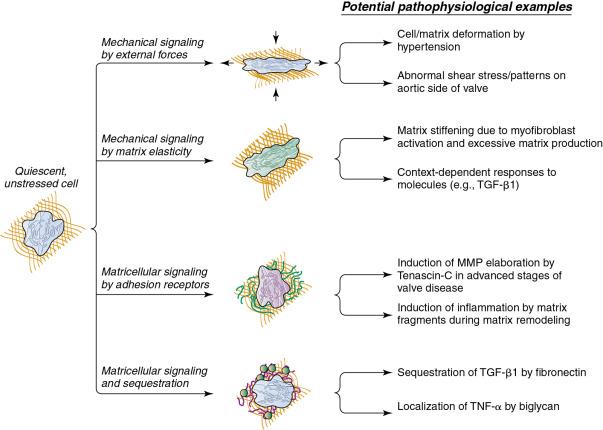
Matricellular signaling refers to induction of signals within the VIC by direct interactions with ECM components. An example of matricellular signaling is the interaction between VICs and tenascin C. Although tenascin C levels are low in normal valves, expression is markedly upregulated, and location shifts from the subendothelium to the valve interstitium as the severity of calcific aortic valve disease progresses. It has been suggested that matricellular signals initiated by tenascin C upregulate MMP expression and alkaline phosphatase (ALP) activity in VICs. ,
Matricrine signaling refers to the ability of the matrix to modulate the bioavailability and binding of growth factors through their sequestration and localization. Examples of matricrine signaling are the regulation of latent transforming growth factor-β (TGF-β) assembly, the storage by fibronectin and binding of TNF-α by biglycan, and the release of proinflammatory and procalcific elastin degradation products by cathepsins. Upregulation of ECM molecules may play a key role in the localization of profibrotic and proinflammatory molecules to sites of calcification and injury in the valve.
Mechanical signaling refers to changes in external mechanical forces that are ultimately transmitted to aortic VICs by the ECM. Hypertension, which is a major risk factor for development of calcific aortic valve disease, increases myofibroblast activation and appears to accelerate the differentiation of cells to an osteoblast-like phenotype. , Detailed studies of changes in VIC biology in experimental models of hypertension (e.g., with or without genetic alterations in ECM proteins) are needed to understand the role of the ECM in the integration of physiologic and biochemical cues in vivo.
The stiffness of the ECM can have a profound effect on the differentiation of cells in response to various lineage-directing cues. Reports clearly demonstrate that matrix stiffness is an independent determinant of cellular differentiation and determines whether cells undergo apoptosis or osteogenesis after specific stimuli (e.g., TGF-β). , , Collectively, increases in matrix stiffness with progression of calcific aortic valve disease are likely to perpetuate osteogenesis, apoptosis, and calcification in an independent manner. This may be a major mechanism underlying the propagation phase of the disease process whereby calcification appears to beget further calcification.
Although inflammation and lipid deposition are implicated in the initiation of aortic stenosis, calcification appears to be the key process driving progressive valve narrowing and disease progression in the propagation phase. Atherosclerotic risk factors do not predict disease progression; rather, it is most closely associated with markers of valve calcification, whether measured using echocardiography, computed tomography, or positron emission tomography. When devising strategies to slow disease progression in aortic stenosis, calcification is a key target.
The propagation phase of aortic valve calcification appears to be a closely regulated process that depends on the influence of osteoblast-like cells that develop an osteogenic phenotype. Approximately 20% of stenotic aortic valves have evidence of bone matrix at the time of valve surgery. Histologically, the bone matrix has been associated with cells that resembled osteoblasts and osteoclasts ( Fig. 3.5 ). This finding suggests that this pathologic process is organized and regulated and that it may also be modifiable with targeted therapy.
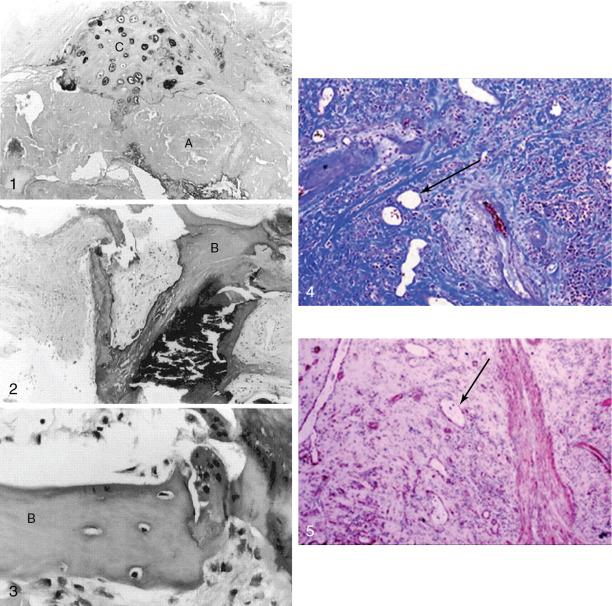
The similarity between skeletal bone formation and aortic stenosis is further supported by gene-profiling studies showing increased expression of the runt-related transcription factor 2 gene (RUNX2) (i.e., core-binding factor subunit α1 [CBFA1] ) in aortic valve tissue, an essential component of osteoblastic differentiation and regulation. Subsequent work demonstrated upregulation of several other ECM proteins closely involved in osteoblastic processes, including osteopontin and bone sialoprotein, which have complex roles that include facilitating osteoblast attachment to bone matrix.
The source of osteoblast-like cells remains unclear. Although several cell types in native valve tissue can undergo differentiation into an osteoblastic phenotype in vitro, the most plausible candidate is the VIC. Fibroblasts can become activated by a variety of cell signaling pathways, including angiotensin II, to adopt the myofibroblast phenotype demonstrated by VICs and characterized by α-smooth muscle actin (α-SMA) expression and increased type I collagen synthesis. The percentage of VICs increases in calcific valve disease (up to 30%), and VICs are thought to develop an osteoblast-like phenotype. ,
The transition of the VIC to an osteogenic phenotype may be the key step in establishing the propagation phase of the disease, and it is regulated by a growing list of signaling molecules and complex pathways. In the early stages, macrophage-derived proinflammatory cytokines appear to be important. , , Later, self-perpetuating, procalcific pathways dominate, including bone morphogenetic protein, WNT/β-catenin, and receptor activator of nuclear κ-B (RANK)/RANK ligand (RANKL)/osteoprotegerin (OPG) pathways. A vicious cycle of calcification producing osteogenic differentiation and further calcification appears to become established, in part driven by increased mechanical stresses and valvular injury related to the calcium deposition ( Fig. 3.6 ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here