Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Molecular testing for inherited disorders is one of the most rapidly growing areas of molecular pathology owing to the plethora of disease genes discovered through the Human Genome Project and the introduction of exome and genome sequencing.
The mutations for single-gene disorders, whether dominant or recessive, can be detected by a variety of molecular diagnostic techniques, either specific to the mutation in question, if it is known, or by a panel of the more common mutations or by comprehensive gene sequencing if the mutation is not known.
Certain disorders, such as cystic fibrosis, have sufficiently high mutation carrier frequencies that they have become targets for large-scale population screening programs. With the advent of massively parallel (“next-generation”) DNA sequencing, the potential mutation, gene, and disease targets for individual, couple, or population screening have greatly expanded.
Late-onset dominant disorders, such as Huntington disease and familial cancers, are appropriate targets of presymptomatic testing provided that sufficient attention is paid to the associated genetic counseling and ethical concerns.
Next-generation sequencing has revolutionized this field in the last few years, with exome or genome sequencing, or large gene panel testing, tending to replace or supplement single-gene testing. This approach has been especially fruitful in providing answers to patients with ultra-rare or undiagnosed disorders.
Ethical issues raised by each of these applications involve genetic privacy, informed consent, pregnancy termination, reporting of off-target findings, duty to reanalyze and recontact test subjects, potential stigmatization, and theoretical risk of insurance or employment discrimination.
Diagnostic molecular genetics shows promise of becoming the most powerful diagnostic and screening tool of the 21st century, spurred on by the introduction of high-throughput DNA sequencing technologies and the ever-growing description of gene-disease associations in the human genome. With this progress has come the recognition that virtually all diseases, including neoplastic and even infectious ones, have some genetic component, with the result that the clinical utility of this subspecialty can only continue to expand. Moreover, its unique capability to diagnose disease both prenatally and presymptomatically should confer on it a primary role in preventive medicine, a focus of increasing urgency in the present era of medical care cost containment. This goal will truly be brought home when researchers identify the genetic causes of the so-called “common complex” diseases: atherosclerosis, diabetes, hypertension, dementia, and so on. Even beyond that, diagnostic molecular genetics leads naturally into therapeutic molecular genetics because essentially the same normal gene sequences used to detect molecular genetic defects by deoxyribonucleic acid (DNA) hybridization could theoretically be used to correct such defects by gene replacement therapy or gene editing. Such therapies, in turn, will come under the purview of the molecular diagnostics laboratory, which will have the responsibility of confirming proper insertion and appropriate expression of the replaced or edited gene.
Yet such progress does not come unencumbered by appreciable obstacles. Aside from the considerable technical sophistication, expense, and complexity of these procedures, they are inextricably bound up with a number of thorny ethical dilemmas. Dissecting a patient’s most fundamental constitutional makeup and the inborn errors therein raises problematic questions about genetic discrimination, stigmatization, ethnic differences, privacy, informed consent, and confidentiality. Since at the molecular genetic level everything becomes a preexisting condition, the very definition of insurability may need to be revised. Instances of insurance and employment discrimination as a result of genetic testing, although rare ( ), have been reported ( ). It is reassuring that in the United States, where privately insured health care is most vulnerable to such abuses, we now have federal legislation—the Genetic Information Nondiscrimination Act (GINA)—prohibiting discrimination in health insurance and employment based on genetic test results, although certain limitations still remain ( ). Furthermore, discovery of any such heritable mutations in an individual has profound implications far beyond the immediate patient who is the target of the DNA test (the proband ), extending to all the other members of that person’s family, none of whom may have consented to exploring or revealing this type of information. Indeed, with the almost unlimited power of DNA testing afforded by amplification techniques such as the polymerase chain reaction (PCR) and high-throughput genomic analysis technologies (see Chapter 69, Chapter 70, Chapter 80 ), it becomes quite easy to perform genetic analysis without the patient’s consent or even knowledge because the testing can be done on minute portions of tissue or fluid samples obtained for other unrelated purposes. Prenatal diagnosis—and, by extension, preconception genetic carrier screening of couples—has become caught up in the fevered ethical and religious debates over abortion. In addition, gene therapy/gene editing, despite general consensus that it should be directed only at somatic rather than germline cellular targets (with high-profile violations of this rule already occurring) ( ), raises the specter of eugenics among those who do not have to remember back all that far to times when such notions were not only accepted but actively espoused.
Much of diagnostic molecular genetics involves the assessment of risk for occurrence or recurrence of a disorder in an individual or family. For reasons described more fully later in this chapter, the test results obtained are often expressed not in terms of a numerical concentration or as a yes/no answer, but rather as a probability, which sometimes is derived by multifactorial Bayesian analysis ( ) or a complex variant interpretation algorithm ( ). The accurate and meaningful conveyance of such uncertainties to patients and even referring physicians can be quite difficult and time-consuming. For this reason, this area of laboratory medicine, perhaps more than most others, requires very close communication between the laboratory and the referring clinician or genetic counselor. In fact, some of these tests, particularly the emotionally charged predictive ones, should be ordered only through a medical geneticist or genetic counselor or through some alternative method of informed pretest counseling. Many large academic and commercial reference laboratories specializing in this type of testing employ their own genetic counselors on staff as a further safeguard to ensure appropriate test selection and communication with primary care physicians who may not be well-versed in these matters.
With so many genetic disease genes, loci, and mutational mechanisms known, diagnostic molecular genetics must take advantage of the entire spectrum of modern molecular biological techniques available. These include, among others (see Chapter 68, Chapter 69, Chapter 80 ):
PCR
Southern blotting
Allele-specific probe hybridization
DNA sequencing (Sanger method)
Real-time PCR
Nucleic acid microarrays
Multiplex ligation-dependent probe amplification (MLPA)
Mass spectrometry
Massively parallel (next-generation) DNA sequencing (NGS)
RNA sequencing
To make such tests practical and affordable, a number of multiplexing strategies for simultaneous mutation detection have been devised. All of these involve some compromise as to overall test sensitivity. Indeed, the field of molecular genetic testing tolerates, by necessity, a number of screening tests with clinical sensitivities noticeably below those that would be considered acceptable in other areas of the clinical laboratory. The decision of just how low the acceptable sensitivity cutoff should be often comes down to public health considerations. Most geneticists have reasoned that, at least for screening tests, the potential public health benefits of offering an inexpensive and technically feasible test of admittedly suboptimal sensitivity outweigh the arguments for withholding it, as long as sufficient education and counseling are provided to patients so that they understand the residual risk inherent in a negative test result. It is important to keep in mind that this discussion is about clinical sensitivity, not analytical sensitivity. It is assumed (as validated by the laboratory) that the test is capable of detecting a given mutation whenever it is present; it is just that many rarer mutations will not be targeted by the assay, so that some proportion of carriers will be “missed”—a sort of “clinical false-negative” ( ). Direct mutation tests were simplified immeasurably by the advent of PCR in the mid-1980s. Through the judicious choice of primers, this technique allows the laboratory to home in on the precise mutation of interest, or a “hot spot” within a gene containing several possible mutation sites, using minute amounts of starting material. Once the region containing the suspected mutation is amplified, it can be analyzed by gel or capillary electrophoresis, sequencing, or DNA probe hybridization. For a deletion or insertion that would be expected to alter the length of the amplicon, accurate molecular sizing of the PCR products by electrophoresis will be sufficient. For point mutations, a common approach is to hybridize the PCR products with allele-specific oligonucleotide (ASO) probes, short DNA fragments that are precisely complementary to either the normal or mutant target sequence. As discussed in Chapter 70 , if the hybridization is performed under sufficiently stringent conditions, target DNA containing the mutation will hybridize only with the mutant probe and vice versa for wild-type target DNA. Several mutation hot spots in a gene can be amplified together by multiplex PCR. As a variation on this approach, any number of allelic probes can be spread out on a solid support for subsequent hybridization with the specimen DNA (or amplicons) in the form of a microarray or in suspension on microbeads. Lastly, a number of commercial reagents and instruments are available that detect point mutations by differential probe/quencher hybridization or by capillary electrophoresis and other sophisticated techniques, as described in Chapter 68, Chapter 70 .
Disorders caused by expansion of a trinucleotide (or other short tandem) repeat are typically diagnosed by PCR followed by sizing on a capillary electrophoresis instrument and observing a larger-than-normal target DNA fragment. Disorders caused by large deletions may be diagnosed by Southern blot by observing loss or decrease in size of a target fragment, or by PCR through loss of a product normally amplified from that site or appearance of a new “junction” fragment, or by MLPA or microarray. It should be noted that a weakness of the newer next-generation DNA sequencing (NGS) techniques is their inability to identify either repeat expansions or sizeable deletions (except for deletions on the X chromosome in a male), though this deficiency is starting to be overcome, especially through whole-genome sequencing ( ).
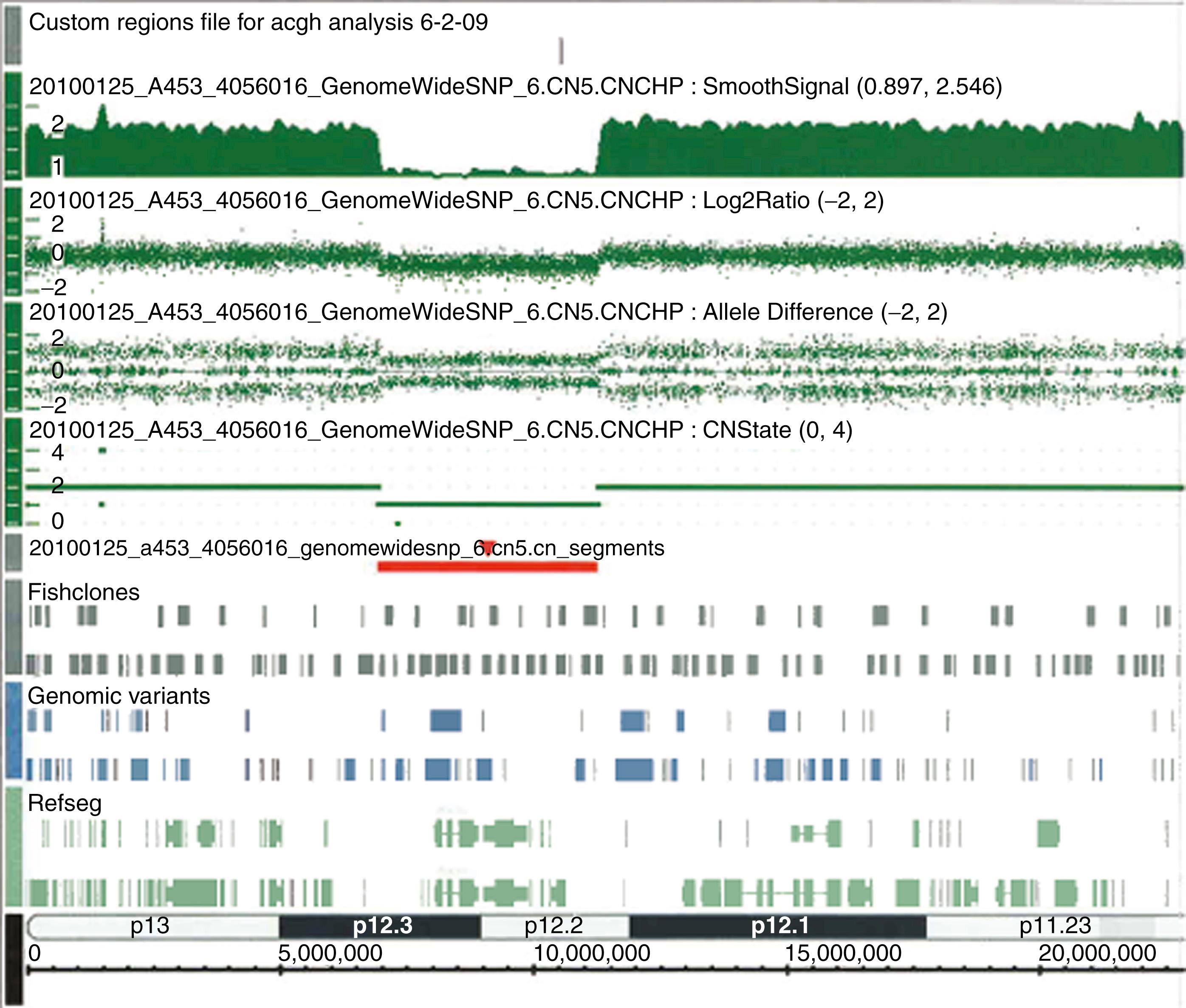
When the diagnostic investigation must go beyond a single gene, in cases of broad differential diagnosis, we turn to high-density oligonucleotide microarray platforms or NGS. The former was the first to enter routine use in clinical genetics in the form of array comparative genomic hybridization, now usually called chromosomal microarray (CMA). This technique employs a high number (500,000 to > 5,000,000) DNA probes on the array, chosen so that their complementary genomic sequences span the entire genome at regular intervals. Total genomic DNA from the patient is compared by hybridization to the array against what a “normal” total genomic control sample would produce (i.e., no pathologic gains or losses of hybridization signal). If the patient’s DNA contains a deletion on a certain chromosome, this will be indicated by a decrease or absence of hybridization signal to the probes spanning that region ( Fig. 72.1 ). Conversely, if the patient’s DNA contains a duplication (insertion) of a chromosomal region, an excess of hybridization signal will be seen for that region. The resolution for detecting these copy number variants (CNVs) is much finer than can be achieved by classic karyotype analysis under the light microscope and is continually improving as ever greater numbers of probes at ever diminishing base-pair intervals are added to the arrays. As such, it has rapidly become a predominant technique for detecting deletions/duplications in patients with congenital problems that do not readily suggest a particular known genetic syndrome, including those with nonspecific physical malformations/dysmorphisms, developmental delay, and/or autism. In fact, it is now considered standard of practice for these indications instead of ordering a karyotype ( ; ). However, it does have some limitations. First, because all it does is examine differences in hybridization intensity across the total genomic DNA, balanced translocations and inversions with no gain or loss of genetic material will not be detected. Second, all human genomes contain a great many CNVs that are nonpathogenic (i.e., benign). Some of these are well-known polymorphisms and, thus, can be discounted as etiologic if observed in a patient. However, many others are not yet known or studied extensively, producing the challenging “CNV of uncertain clinical significance.” Aside from using intuition based on the particular genes encompassed by the deletion or insertion, testing of the parents may also be helpful. If a normal parent carries the same deletion, it is less likely to be pathogenic (though there are exceptions). More detailed discussion of microarray technology can be found in Chapter 70 .
Of course, the ultimate gold standard for identifying all possible mutations would be whole-genome sequencing. It took 13 years to completely sequence the consensus human genome under the Human Genome Project using conventional Sanger sequencing platforms (see Chapter 68 ). We now have a new generation of automated DNA sequencers based on radically different chemistries involving sequencing of millions of short fragments of the patient’s genome in parallel, which are then assembled into the full genome by analytic and bioinformatic software. These instruments are capable of sequencing billions of bases per day at relatively reasonable cost (with costs diminishing all the time), at last putting the whole human genome in reach for routine analysis. At present, the major clinical application of this technology in genetics is for the diagnosis of syndromic-appearing conditions for which standard single-gene and other clinical laboratory tests have been unrevealing (see later discussion for detailed description of this process). It is also being applied to oncology, with NGS of tumor DNA to detect so-called “druggable” somatic mutations (see Chapter 78, Chapter 79 ). Further discussion of NGS in present and future applications can be found in Chapter 80 .
To some extent, the choice of technique will also depend on the application or clinical indication. In medical genetics, these applications fall into five major areas: carrier screening, newborn screening, diagnostic testing, presymptomatic DNA testing, and prenatal testing.
Carrier screening is the term applied to detection of recessive mutations in healthy individuals for purposes of genetic counseling and family planning. This application is further subdivided into screening of those individuals with a family history of the disorder and population-based screening of large numbers of individuals who have negative family history but who may be at risk for the disorder because of its prevalence within their ethnic group or in the population at large. In either case, the ultimate purpose is to identify couples at risk (i.e., both the father and mother are heterozygous for mutations within the gene) who would then have a 25% chance of having an affected child with each pregnancy. The testing strategies chosen for the two groups will differ, however. A person whose sibling has the disorder is at much higher risk of being a carrier than someone in the general population. That person may therefore warrant more aggressive testing (e.g., screening for a greater number of mutations or possibly complete gene sequencing) than would be cost-effective for the general population. On the other hand, access to the affected sibling’s DNA may allow prior identification of the familial mutation, which would render subsequent testing of other family members much easier and straightforward. Population-based screening, in contrast, typically strives to keep the testing procedure as rapid and inexpensive as possible, focusing on perhaps a few of the more prevalent mutations, sacrificing clinical test sensitivity for cost-effectiveness and expediency. With a negative family history, there are no affected family members to make either single-site mutation testing or linkage analysis an option. In recent years, NGS has allowed for carrier screening in many more genes (into the hundreds) at reasonable cost. This approach is agnostic to the long-standing previous indications for targeted carrier screening: positive family history and/or ethnic risk. While uptake has been high in prenatal clinics, there remains some controversy as to its utility. On the positive side, it will detect mutations in genes for ultra-rare disorders that would not have been cost-effective to screen in large populations before, thus offering the opportunity for couples to avoid the birth of a child with a completely unexpected disorder for which they did not know they were at risk ( ). On the negative side, the addition of diseases and genes to construct the expanded panels has been rather arbitrary, with a number of diseases included for which little is known of the natural history, severity, or genotype-phenotype associations of the many novel sequence variants that may be detected ( ). In addition, the inclusion of so many genes means that a significant proportion of the tested individuals (up to 30% or more) and couples will be positive for something ( ), creating a downstream burden on genetic counseling resources.
Like population-based carrier screening, newborn screening aims to identify relatively prevalent (as genetic diseases go) inherited defects in otherwise asymptomatic individuals. The most important disease targets—such as phenylketonuria, galactosemia, sickle cell disease, and cystic fibrosis (CF)—are likewise autosomal-recessive disorders. In the case of newborn screening, however, the goal is to ascertain affected babies early in life so that treatment (dietary or pharmaceutical) can be initiated before irreversible damage occurs. Currently, molecular genetic methods in this setting are employed mainly as a backup for confirmation of positive results ascertained by less expensive and more comprehensive biochemical or enzymatic methods, but this situation could reverse itself as molecular methods become increasingly cost-effective, high-throughput, and comprehensive. Ascertaining the actual mutations in the affected gene can offer additional information as to potential severity and drug response beyond the mere diagnosis produced through biochemical methods. Moreover, as NGS becomes increasingly less costly, it is not such a stretch to predict that all newborns may someday undergo a full-genome analysis as a matter of course—though whether and which results get reported out at that age will be a subject of intense debate.
Diagnostic genetic testing is, by definition, performed on a symptomatic individual. Because the single-gene DNA tests are absolutely disease-specific and the diseases themselves quite rare, these procedures do not cast a wide enough net to be used for extensive differential diagnosis. The symptoms must be sufficiently suggestive of the disorder in question to justify ordering the test. Also, one must weigh the DNA test against more traditional methods with regard to cost, convenience, and utility. For example, hemoglobin (Hb) electrophoresis may be more convenient and comprehensive for sorting out a suspected hemoglobinopathy than the specific DNA test for the sickle cell disease or thalassemia mutations alone. On the other hand, molecular testing may be more advantageous for early or atypical clinical presentations. For example, molecular testing CF mutations can be performed in the newborn period when traditional sweat chloride analysis is either inconvenient or unreliable. In such cases, it is also important to determine that the mutation spectrum of the test represents the appropriate demographic. If not, a “negative” test may not be truly accurate. DNA tests also have the advantage of working well postmortem, when classic biochemical analytes can no longer be assessed.
Presymptomatic DNA testing is applied primarily to late-onset dominant disorders, in which the offspring of an affected parent are aware that they are at 50% risk of having inherited the disease gene and desire to know their status before its clinical onset to make informed reproductive, employment, and lifestyle decisions or to initiate surveillance or preventive interventions. The prototypic disorders in this group are Huntington disease and the heritable cancer syndromes, although such diseases as neurofibromatosis, Marfan syndrome, adult polycystic kidney disease, and tuberous sclerosis are also relevant. This sort of testing has been the most problematic, from a psychosocial and ethical standpoint, of any in diagnostic molecular genetics, with a risk of severe adverse consequences of results reporting, including insurance and employment discrimination and even suicide. Because of this, established testing protocols include stipulations for proper informed consent, concurrent clinical assessment, extensive pretest and posttest genetic counseling, and psychosocial support ( ; ).
Finally, there is the clinical application, which accounts for a large proportion of medical genetics services: prenatal testing , or the detection of genetic disease in the fetus. With a few exceptions (e.g., hydrops fetalis in homozygous β-thalassemia, thanatophoric dwarfism and other skeletal dysplasias, and type I osteogenesis imperfecta), most Mendelian disorders, especially inborn errors of metabolism, are not expressed either visibly (by ultrasound) or biochemically in the fetus. Thus, predictive diagnosis can be made only at the DNA level. Even for those disorders that might be detected biochemically, DNA often proves to be a far more accessible substrate, from an obstetric point of view, than the affected protein products or metabolic substrates. Whereas molecular analysis can be performed on minute amounts of amniotic fluid or chorionic villus samples collected by routine methods, even if obtained for other purposes, unless the protein product is expressed in fibroblasts (and thus amniocytes), biochemical analysis will require invasive biopsy of deep fetal tissues or fetal blood sampling. For example, assay of phenylalanine hydroxylase activity to diagnose phenylketonuria would require fetal liver biopsy, and quantitation of dystrophin to diagnose Duchenne muscular dystrophy (DMD) would require fetal muscle biopsy.
The primary objective in prenatal diagnosis is the identification of an affected fetus in a timely manner so that a practical option of pregnancy termination can be offered to the couple. Even though some may argue an advantage for obtaining diagnosis prenatally so that therapy can be instituted promptly at birth or for psychological reassurance of a couple if the fetus is found to be unaffected, it may be difficult to justify the risk (albeit low) of miscarriage from amniocentesis and chorionic villus sampling performed for these other purposes. For an affected fetus, unless one intends to initiate therapy in utero , provisional treatment at birth while awaiting confirmatory neonatal testing is perfectly acceptable.
Although prenatal genetic counseling is always nondirective, with moral and/or religious objections to abortion respected, both the clinician and the DNA testing laboratory have a legitimate right and, indeed, responsibility to question the appropriateness of a prenatal test request, with its attendant risk and expense, from a couple for whom termination is not an option (the same would apply to requests coming too late in pregnancy for termination to be performed). It is because of these problems that invasive prenatal testing is not offered as a general population screening tool in women with no family history or known carrier status for the disorder in question. The power of PCR to enable single-cell genetic analysis has opened the way for preimplantation diagnosis, usually approached by performing in vitro fertilization and microdissection of a single blastomere from the early embryo. This strategy, initially applied to selected cases at risk for CF ( ) and other disorders, could potentially be offered to any at-risk couple for whom abortion is not an option, although it is not without its own ethical (and economic) considerations. More recently, it has begun to be used to diagnose adult-onset diseases such as familial breast/ovarian cancer ( BRCA1 and BRCA2 genes), which, because they are late onset, incompletely penetrant, and potentially treatable, would otherwise raise uncomfortable objections to traditional amniocentesis and pregnancy termination among some groups ( ).
Despite all of these medical and moral dilemmas, when performed in appropriate circumstances, prenatal molecular genetic testing can offer at-risk couples, many of whom have already suffered the trauma of at least one affected child, one of the most valuable and life-changing services in all of clinical medicine.
Whereas the DNA analysis techniques discussed in this chapter for diagnosis of genetic disease are generally the same as those used for molecular diagnosis of cancer or infectious diseases, their application in the former has revealed a number of unusual phenomena that one must keep in mind when dealing with particular hereditary disorders. Some of these phenomena have been known since Mendel’s time but can now be understood mechanistically at the DNA level. Others have emerged much more recently as unexpected by-products of the molecular dissection of specific disease genes.
Few genetic disorders are associated with a single mutation consistently identified in all affected cases (e.g., the missense mutation in codon 6 of the β-globin gene causing sickle cell disease). The vast majority of genetic disorders can be caused by more than one—sometimes hundreds or thousands—of different mutations within the disease gene (e.g., the CFTR gene of CF), and sometimes even by more than one gene (e.g., the TSC1 and TSC2 genes of tuberous sclerosis or the BRCA1 and BRCA2 genes of familial breast/ovarian cancer). Obviously, identifying the causative mutations in such disorders is technically much more difficult or sometimes impossible. A corollary of such molecular heterogeneity is that not all of the mutations will produce equally severe disease. Some may cause only mild forms or related syndromes, with little resemblance to the classic phenotype (e.g., isolated congenital absence of the vas deferens caused by certain mutations in the CFTR gene, or either multiple endocrine neoplasia or Hirschsprung disease caused by different mutations in the RET gene). All of this variability adds greatly to the complexity of genetic counseling and genetic testing.
Penetrance refers to the proportion of individuals who, having inherited a mutant disease gene, will actually display the disease phenotype. Usually applied to dominant disorders, it can produce the striking appearance of generation-skipping in disease pedigrees. This can complicate both molecular diagnostics and genetic counseling, because it may not be clear whether the proband inherited the disease from a parent or instead represents a new mutation in the family. It is a feature of such relatively common genetic disorders as Marfan syndrome and neurofibromatosis.
Variable expressivity refers to the appearance of different signs, symptoms, and severity of a disorder in individuals inheriting the same mutation(s). Like penetrance, it is probably a reflection of differential gene effects within dissimilar genetic backgrounds (in other words, the modulation of phenotypic expression by other nonallelic or modifier genes). It, too, makes ascertainment and counseling difficult, and raises ethical challenges in considering pregnancy termination for diseases of variable and unpredictable severity (see earlier discussion of expanded carrier screening).
This unusual cause of a recessive single-gene disorder was first discovered in a CF patient, only one of whose parents was a carrier ( ). By DNA haplotyping using polymorphic markers, it was shown that the patient had inherited two copies of the carrier parent’s chromosome 7 containing the mutant CFTR gene and no chromosome 7 from the other parent. The phenomenon has since been observed in other cases of CF and diseases involving other chromosomes as well. For some diseases, such as Prader-Willi and Angelman syndromes (see later discussion), the incidence of uniparental disomy approaches that of classic mutation mechanisms in the molecular pathogenesis, justifying routine testing for this phenomenon (typically by haplotyping the pair of chromosomes for shared polymorphic markers or, in some cases, by methylation analysis).
Imprinting refers to the differential expression of a gene in an offspring depending on whether it was inherited from the mother or the father, or sometimes on other epigenetic influences. Some genes are expressed—or, conversely, turned off—only when they pass through the oocyte lineage and others only when they pass through the spermatocyte line. If an individual inherits the normal allele through the nonexpressing parental line, it cannot counteract a recessive mutation inherited from the other parent. In at least some cases, the molecular mechanism appears to be differential methylation of chromosome regions and regulatory elements. This is the basis for both the deletional and uniparental disomy cases of Prader-Willi and Angelman syndromes, as discussed in more detail later ( ).
Anticipation refers to a progressive increase in severity and/or decrease in age of onset of a genetic disorder in subsequent generations of a family. It is typically associated with the trinucleotide repeat disorders, such as myotonic dystrophy and Huntington disease, in which the increasing severity can be correlated with further expansion of the repeat region. In the former disease, especially severe cases with childhood or infantile onset, they have been born to affected mothers, while in the latter disease the phenomenon occurs solely with affected paternal transmission, invoking a parent-of-origin effect similar to imprinting ( ). It is for these reasons that accurate molecular sizing of trinucleotide repeat lengths is so important for diagnosis, prognosis, and genetic counseling in these disorders.
Epigenetic changes are heritable but potentially reversible changes in gene expression that do not represent a change in the sequence of the cell’s genomic DNA. The most striking examples of epigenetic inheritance are genomic imprinting (discussed previously) and mammalian X-chromosome inactivation, both involving transcriptional silencing of genes by methylation of cytosines at CpG dinucleotides. The methylation status of DNA is maintained following DNA replication by methylases, which act on hemimethylated double-stranded DNA to methylate the newly synthesized DNA strand as well. The process is perpetuated indefinitely in succeeding cell divisions. We now know that de novo methylation of CpG doublets can occur in the promoters of some tumor suppressor genes, silencing these genes and in essence constituting one or both of the “hits” in a tumor suppressor gene that leads to tumor development ( ).
Another category of epigenetic inheritance that is less clearly understood involves the property of some proteins to influence the conformation of newly synthesized or assembled proteins in a self-perpetuating manner. The most notable examples in mammals are the prion diseases, responsible for scrapie and bovine spongiform encephalopathy in animals and kuru and Creutzfeldt-Jakob disease in humans. The disease-producing prion protein, which may result from a coding sequence mutation in familial cases, is folded into an abnormal conformation and exerts an effect on newly synthesized prions in such a way that it perpetuates and proliferates the conformational abnormality that produces disease ( ).
Reference has already been made to the application of carrier screening for recessive mutations on a population-wide basis. To justify, from a public health standpoint, the effort and expenditure required to perform a DNA test on thousands or millions of people, the incidence of the disease must be sufficiently high either in the whole population or in the particular racial or ethnic groups being selected for screening. For any autosomal-recessive disease of appreciable incidence, the law of Hardy-Weinberg equilibrium predicts that the carrier frequency will be a good deal higher than the prevalence of affected individuals. In addition, the candidate disease target must be sufficiently severe and/or amenable to some medical intervention upon identification. Several disorders appear to fit these criteria. Mutations associated with hereditary hemochromatosis and activated protein C resistance (factor V Leiden) are found in 5% to 10% of the white population, while the carrier frequency of the sickle cell mutation approaches 10% in the African American population. Unfortunately, controversies over disease penetrance in the first two and complex socioeconomic issues in the third have limited the application of these genes to screening ( ; ). Screening for thalassemia in Mediterranean and Asian populations, and for a panel of recessive disorders such as Tay-Sachs disease and Gaucher disease in the Ashkenazi-Jewish population, is similarly justified by allele frequencies in the target group. CF mutations, while of lower frequency, potentially place such a large majority of North American couples at risk that they have been chosen as the first target for general molecular genetic population screening in the United States (see later discussion). Other disease targets proposed for large-scale screening include spinal muscular atrophy, fragile X syndrome, and hereditary hearing loss. This migration of molecular genetic screening out of the realm of rare or esoteric diseases and into the setting of common traits will have a profound effect on preventive medicine and public health, and will continue to drive new developments in DNA test automation in the coming years.
Unique among molecular pathology tests—and, indeed, among clinical laboratory tests in general—molecular genetic tests possess the ability to predict future disease in individuals with no signs or symptoms of the disorder at the time of testing. This predictive ability exceeds that imparted to such tests as human immunodeficiency virus antibody screening in individuals with no symptoms of acquired immunodeficiency syndrome, cholesterol levels as a risk factor for future atherosclerosis, or detection of abnormal metabolites by newborn screening. For those tests, one could argue that the serologic, microbiological, or biochemical signs of the disorder are already present, even if the patient does not yet experience any symptoms. Molecular genetic testing, in contrast, enables prediction of future disease even in the absence of any biochemical or physiologic abnormalities: Huntington disease can be predicted decades before there is any brain degeneration, and breast cancer risk years or decades before even a single breast epithelial cell has turned malignant. The potential psychosocial impact of revealing such information to a healthy individual cannot be overemphasized. For that reason, it is highly recommended that predictive or presymptomatic genetic testing be accompanied by pretest and posttest genetic counseling and psychiatric support, as already mentioned. Furthermore, such tests should be restricted to those who truly have an actionable need for them, in the sense that a positive DNA test will prompt some sort of early surveillance or preventive interventions or, at the very least, some guided lifestyle or life-planning changes. Because it is difficult to imagine such conditions existing in the case of a child tested for an unpreventable adult-onset disease, there is a strong convention in the field that healthy children younger than 18 years of age should not be offered predictive genetic tests for adult-onset disorders unless there is a demonstrated preventive intervention that needs to be acted upon in childhood. This will avoid potential stigmatization, discrimination, or adverse psychosocial effects of genetic information that is of no medical use to the child ( ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here