Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Advances in molecular biology are rapidly changing our understanding of skin biology and disease. Increased knowledge is being translated into new molecular diagnostic tests that are transforming the clinical practice of dermatology. Molecular analyses are currently being employed to diagnose genodermatoses , cutaneous infections , melanomas , lymphomas , and inherited or autoimmune blistering disorders . In order to use these tests in a prudent manner, dermatologists nowadays have an even greater need to understand basic concepts in molecular biology. This knowledge is also leading to the development of new targeted therapies, many of which require determination of the molecular basis for disease in order to appropriately implement treatment . In addition, molecular analysis is being used to screen for drug efficacy and susceptibility to adverse reactions to certain medications .
The goal of this chapter is to outline basic concepts and methodologies of molecular biology and to give practicing dermatologists a fundamental appreciation of what their colleagues are doing in the laboratory and how their own clinical practice is changing as molecular approaches are implemented into patient care.
Dermatologists are accustomed to placing a biopsy specimen in formalin followed by paraffin embedding and staining tissue sections with hematoxylin and eosin. However, for molecular analysis, this is just one of several possible starting points for tissue processing ( Fig. 3.1 ). Placing the sample in formalin (for light microscopy) or glutaraldehyde (for electron microscopy) before processing and sectioning allows the sample to be analyzed histologically in a variety of ways, including by routine staining, immunohistochemistry, or in situ hybridization. The advantage of this approach is that the cells are preserved in a very stable fashion, with well-preserved architecture, for later analysis. Disadvantages are that the cells are no longer living and the fixation procedure can place limits on the methods that can be used to analyze DNA, RNA, and protein from cells of interest. Nonetheless, PCR analysis of DNA is increasingly being performed on formalin-fixed, paraffin-embedded tissue, especially for infectious diseases and detection of gene rearrangements in lymphoproliferative disorders.
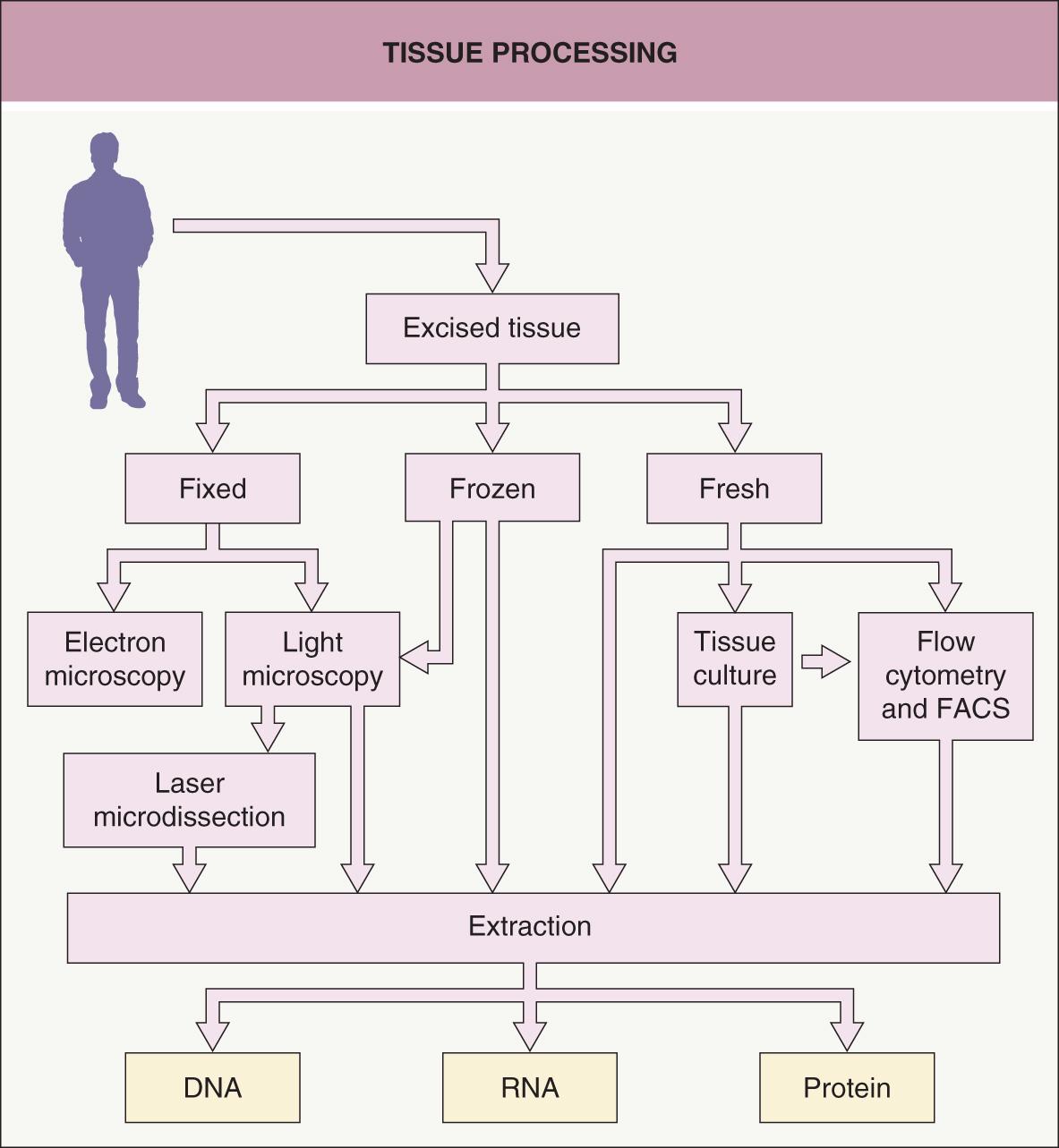
In order to preserve molecules in a more native state, the specimen may be snap frozen. Cryosections prepared from such samples have lower quality tissue architecture than do permanent sections from formalin-fixed, paraffin-embedded tissues, but this form of tissue preservation may allow for better analysis of DNA, RNA, and protein. One may also directly process fresh or frozen tissue with buffers and reagents in order to extract DNA, RNA, and protein from the whole tissue. The advantage of performing extractions from whole tissue is that the DNA, RNA, and protein will be fresh and of high quality. The disadvantage is that the extracts will not be from a pure population of cells.
Another approach is to culture cells obtained from fresh tissue. The desired lineage of cells (e.g. keratinocytes, fibroblasts, immunocytes, endothelial cells) can be propagated in vitro by employing selective culture media and isolation techniques. Culturing cells allows one to obtain more cells than in the original sample, and then the cells can be exposed to various conditions. However, the culturing process may change fundamental characteristics of the cells, so that they do not accurately represent the cells in vivo .
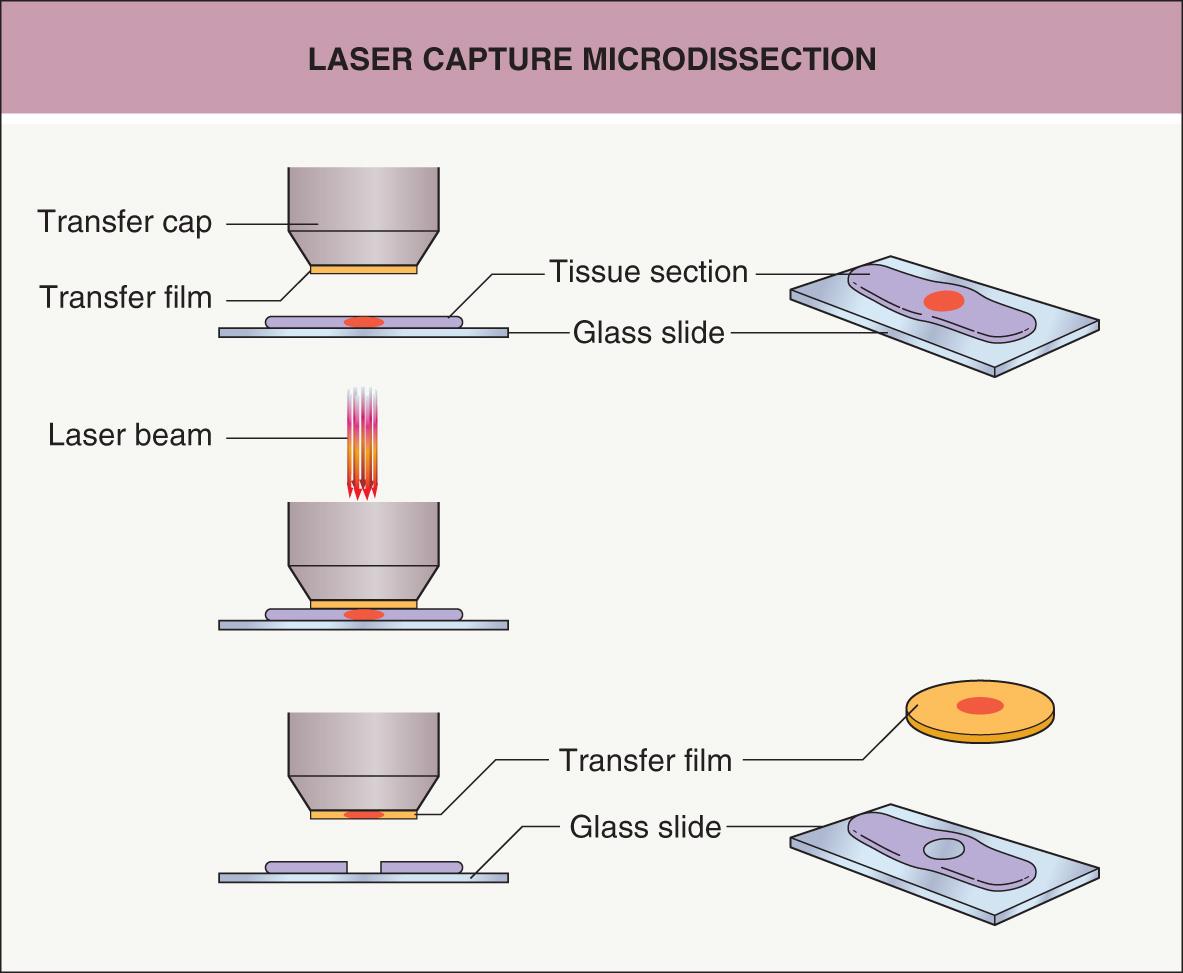
One method for isolating a pure population of cells from a tissue section is to use laser microdissection ( Fig. 3.2 ). A laser is used to retrieve or cut individual cells from a section on a slide while the specimen is viewed with a microscope . An advantage of microdissection is that a very pure population of cells is obtained that has been precisely identified under the microscope. One limitation is that the total number of cells isolated is relatively low, as each cell has to be individually captured by the laser. Consequently, the procedures to extract DNA, RNA and protein from these pure (but few) cells have to be robust.
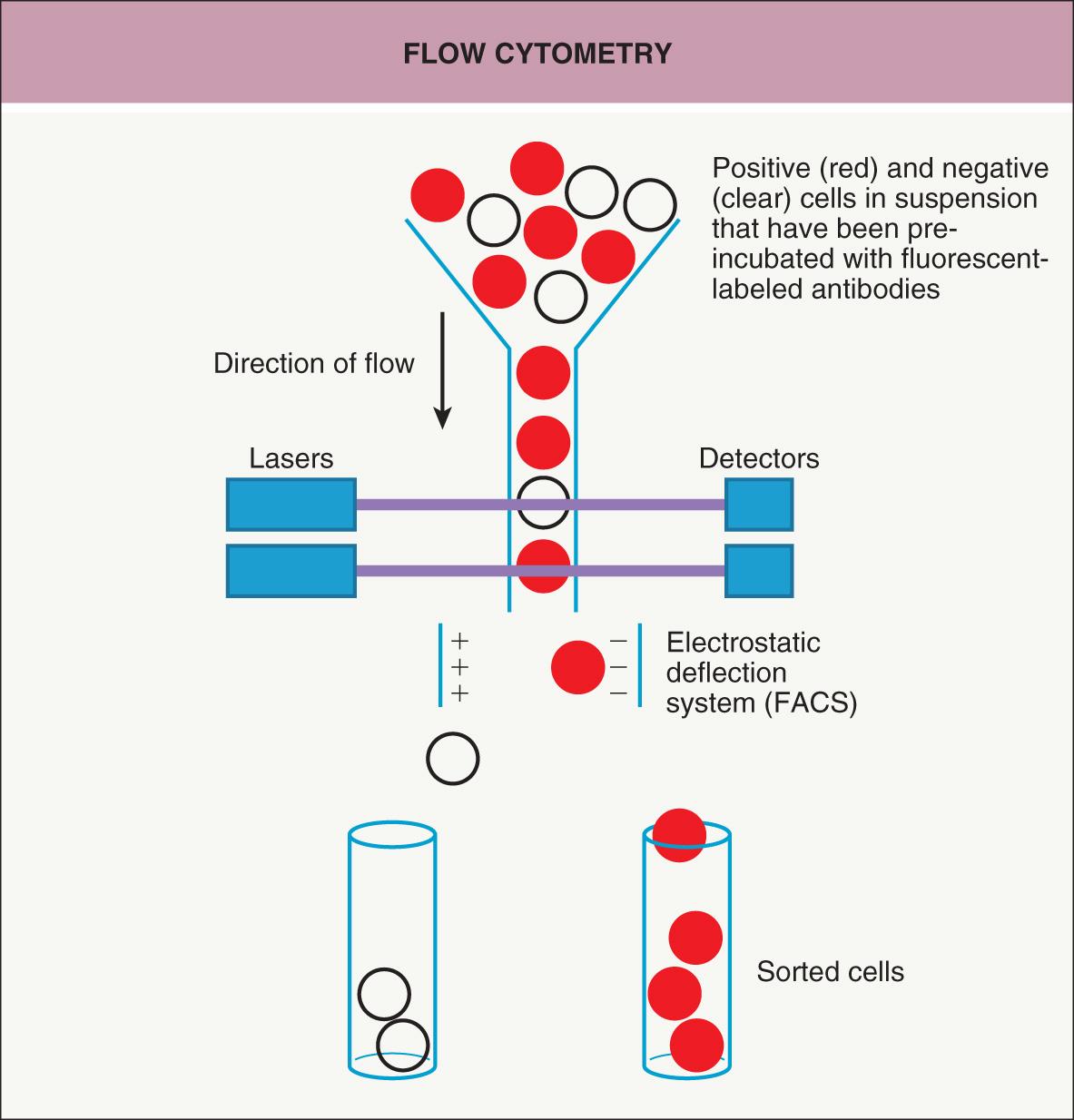
A way to isolate and analyze cells in suspension is to use flow cytometry ( Fig. 3.3 ) . Cells are passed individually in a stream through a set of lasers and electronic detectors capable of measuring multiple parameters for each cell. As an initial step, cells are typically incubated with fluorescent-labeled antibodies that recognize cell-surface markers so that a heterogeneous population of cells can be characterized on a cell-by-cell basis for levels of expression of each marker. Fluorescence-activated cell sorting (FACS), which utilizes an electrostatic deflection system, can also be used to obtain pure populations of cells with specific desired characteristics. Flow cytometry permits the measurement of multiple characteristics of individual cells at a rapid rate. However, with the exception of peripheral blood or bone marrow cells, a significant limitation is that it requires the cells to be in suspension. Recently, the ability to analyze 40 or more parameters for each cell has been accomplished by combining the principles of flow cytometry and elemental mass spectrometry in what is call mass cytometry .
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here