Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
![]() Additional content is available online at Elsevier eBooks for Practicing Clinicians
Additional content is available online at Elsevier eBooks for Practicing Clinicians
While this might come as a surprise to some electrophysiologists, the role of the electrical system of the heart is not to generate nice-looking action potentials (APs) and conduction patterns. Its role is to subserve and control the mechanical function of the heart appropriately. The key elements of this function are illustrated in Figure 62.1A . The electrical system must initiate contraction at a rate and rhythm that is appropriate to the body’s moment-to-moment needs for blood supply to various organs. Appropriate timing of the contraction of different parts of each cardiac chamber (atria and ventricles) needs to be ensured, as does the relative timing of the atria versus the ventricles. The atria serve a primer-pump role and need to contract before the ventricles with an appropriate delay to allow optimal ventricular filling (which is a key determinant of cardiac contractility via the Frank-Starling principle). Finally, excessively rapid and excessively slow rates must be prevented, as these can have disastrous effects on cardiac function.
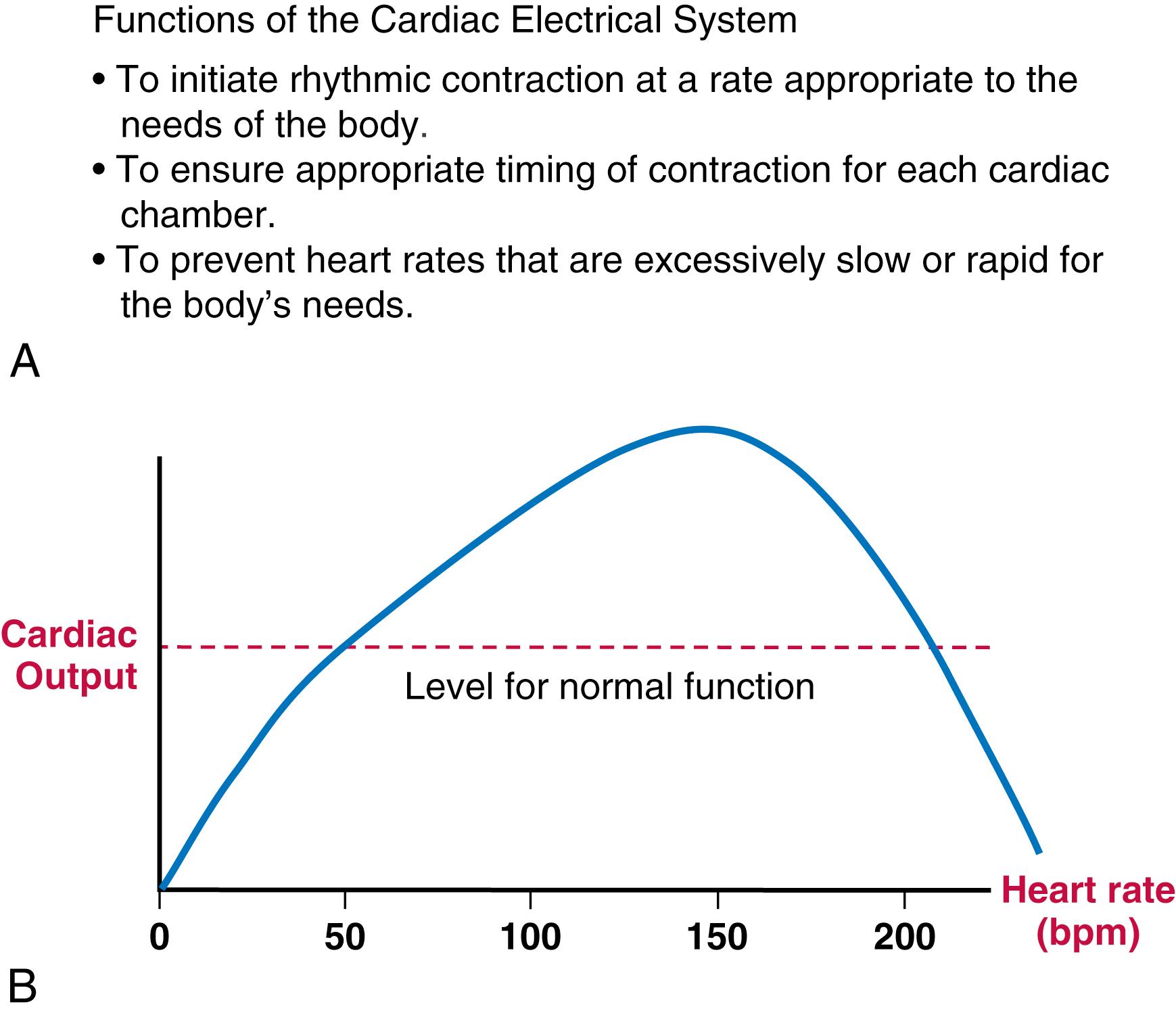
As illustrated by the schematic diagram of the relationship between heart rate and cardiac output in Figure 62.1B , cardiac output is more or less a linear function of the heart rate between about 50 and 150 beats/min (consistent with the relationship cardiac output = heart rate × stroke volume, as long as stroke volume is fairly constant). At heart rates below or above this range, cardiac output can fall precipitously. The key properties underlying the functions listed in Figure 62.1A are automaticity, conduction, electromechanical coupling, and refractoriness. The central cellular characteristic that underlies these properties and cardiac electrical function is the cardiac action potential.
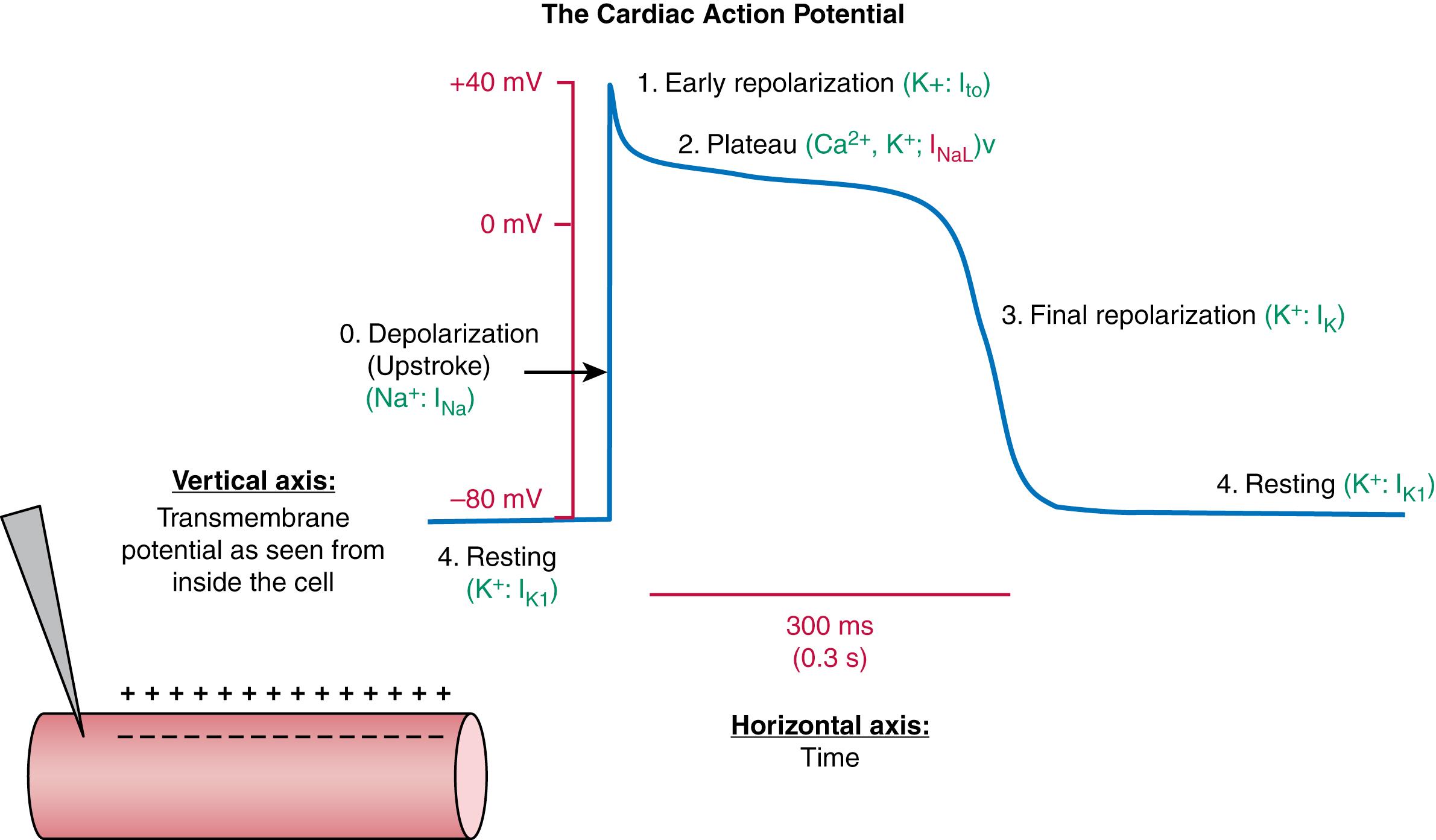
The cardiac action potential (AP) is illustrated in Figure 62.2 . The AP is a graph of the voltage difference between the inside of a cardiac cell and the outside of the cell (as seen from the inside, for example by a fine-tipped electrode placed inside the cell, eFig. 62.1 ), as a function of time. Cardiac cells normally have a negative intracellular resting potential, which for most of the heart (working atrial and ventricular muscle, specialized His-Purkinje conducting system) is about −80 to −90 mV. The exceptions are the sinoatrial (SA) and central atrioventricular (AV) node regions, which have resting potentials between about −50 and −65 mV. The cardiac AP is by convention divided into four phases, the AP-upstroke (phase 0), which depolarizes the cell from its negative resting potential to a potential positive to 0 mV; the initial rapid repolarization phase (phase 1); the so-called plateau (phase 2) in which the AP voltage changes relatively slowly; and the final (phase 3) rapid repolarization phase, which brings the cell back to its resting potential (referred to as phase 4).
| ION | Extracellular Concentration [S] o (mM) | Intracellular Concentration [S] i (mM) | Ratio [S] o /[S] i | E S (mV) |
|---|---|---|---|---|
| Na + | 145 | 15 | 9.7 | +60 |
| K + | 4 | 150 | 0.027 | −94 |
| Cl − | 120 | 5–30 | 4–24 | −83 to −36 |
| Ca 2+ | 2 | 10 −4 | 2 × 10 4 | +129 |
During phase 0, there is a rapid inrush of positively charged Na + ions that mediate depolarization and carry a large inward current (the direction of current is by convention defined by the movement of positive ions), which provides the energy for electrical conduction. Phase 1 sets the voltage level for the subsequent critical phase 2. Phase 2 is the portion of the AP during which Ca 2+ enters the cell and causes a large secondary release of Ca 2+ from the sarcoplasmic reticulum (SR), the main cellular Ca 2+ -storage organelle. The SR Ca 2+ -release rapidly increases free intracellular [Ca 2+ ], which causes cellular contraction and mediates electromechanical coupling. Finally, phase 3 (carried by a rapid egress of K + ) brings the cell back to its negative resting potential. Because the cell cannot be activated by normal means at voltages positive to −60 mV, from when the cell reaches −60 mV during phase 0 until the time that it repolarizes to −60 mV during phase 3 the cell cannot be fired and is “refractory” to activation. Thus, the timing of phase 3 sets the refractory period (RP). The mechanisms by which the crucial AP properties are set depend critically on the function of ion channels, which is discussed in detail below.
Electrical signaling in the heart involves the passage of ions through ionic channels. The sodium, potassium, calcium, and chloride (Na + , K + , Ca 2+ , and Cl − ) ions are the major charge carriers, and their movement across the cell membrane through channel pores creates a flow of current that generates excitation and signals in cardiac myocytes ( Table 62.1 ).
When a semipermeable membrane allows an ion to cross, and the concentration of the ion is different on the two sides of the membrane, a small number of ions will move across the membrane and create an electrical field. For example, consider the case of K + and the cardiac cell membrane. The concentration of K + is maintained (by a complex set of pumps and transporters) at about 150 mM inside the cardiac cell and is about 4 mM outside ( eTable 62.1 ). Cardiac cell membranes are highly permeable to K + at rest, largely because of a specific ion channel, the inward-rectifier K + channel (carrying the current I K1 ). K + tends to leave the cell, down its chemical gradient. However, each K + ion that leaves the cell (unaccompanied by a negative ion, because the cell membrane is largely impermeable to them at rest) leaves the interior of the cell with a slightly negative charge relative to the outside. Equilibrium is created when the chemical force tending to push K + out of the cell is balanced by an equal and opposite electrical force created by the net intracellular negative charge tending to pull the K + back into the cell. The voltage at which these forces balance is called the “equilibrium potential.” In the case of K + , the equilibrium potential is given by the Nernst equation (E = RT/F·ln([K + o ]/[K + i ]), where R is the universal gas constant, T = absolute temperature, F = Faraday’s constant, and [K + o ], [K + i ] = extracellular and intracellular K + concentration, respectively. This value, designated “E K ” for K + equilibrium potential, is about −95 mV. Thus, at rest the high permeability to K + results in a resting potential of about −90 mV (see Fig. 62.2 ), close to the K + equilibrium potential.
As shown in Figure 62.2 , membrane voltages during a cardiac AP vary over the range of approximately −90 to +30 mV. Each phase of the cardiac AP is characterized by its dominant ionic permeabilities (shown in green in Fig. 62.2 ), which determine the direction in which the transmembrane potential moves during that phase. Phase 0 happens when there is a rapid increase in membrane permeability to Na + , and K + permeability falls, making Na + the dominant conductance. At the peak of phase 0 (the “overshoot”), the cell moves close to the Na + equilibrium potential, which is quite positive because in contrast to K + , Na + is more concentrated outside the cell than inside and the Na + chemical force makes it move into the cell, making the interior more positive. Phase 1 is carried by a transient, rapid exit of K + from the cell. Because the calculated reversal potential of a cardiac Ca 2+ channel is +64 mV (assuming P K /P Ca = 1/3000, Ca i = 100 nM, and Ca o = 2 mM), passive Ca 2+ flux is into the cell. During phase 2, the transmembrane potential is governed by balanced permeabilities to Ca 2+ and K + , while during phase 3 a rapid increase in K + permeability offsets the decreasing Ca 2+ conductance and repolarizes the cell. In some cardiac cells (notably His-Purkinje cells) a late Na + current (I NaL ) contributes inward current during the phase 2 plateau.
The changes in ionic permeabilities during the AP are determined by the opening and closing of ion channels. The opening of ion channels allows selected ions to flow passively down the electrochemical activity gradient at a very high rate (>10 6 ions per second). The high transfer rates and restriction to “downhill” fluxes not stoichiometrically coupled to the hydrolysis of energy-rich phosphates distinguish ion channel mechanisms from other ion-transporting structures (pumps and exchangers), such as the sarcolemmal Na + ,K + –adenosine triphosphatase (Na + ,K + –ATPase, which largely maintains the physiological Na + and K + concentrations inside the cell), sarcoendoplasmic reticulum (SR) Ca 2+ -ATPase (SERCA, the SR Ca 2+ -uptake mechanism), or the Na + /Ca 2+ exchanger (NCX, which removes from the cell interior the extra Ca 2+ that has entered during phase 2). Ion channels may be induced to open or close (gated) by extracellular and intracellular receptor-ligands, changes in transmembrane voltage (“voltage-gated”), or mechanical stress (see Table 62.1 ). The most important ion channels in generating the AP are voltage-gated. Ion currents and gating of single ion channels can best be studied by means of the “patch-clamp” technique, in which a glass microelectrode tip is attached to the cell membrane (to record single channel opening), and in some cases a small patch of membrane is torn away to allow electrical continuity between the inside of the cell and the contents of the microelectrode (to measure transmembrane currents). The microelectrode is filled with an electrically conducting fluid that is connected to an amplifier that measures the potential difference between the microelectrode and a reference electrode, generally located in the extracellular fluid.
The permeability ratio is commonly used to define a channel’s ionic selectivity, defined as the ratio of the permeability of one ion type to that of the main permeant ion type. Permeability ratios of voltage-gated K + and Na + channels for other monovalent and divalent (e.g., Ca 2+ ) cations (cations are positively charged ions, so called because they are attracted to a “cathode” or negative electrode) versus their permeant ion are usually less than 1:10. Voltage-gated Ca 2+ channels exhibit a more than 1000-fold discrimination against Na + and K + ions (e.g., P K /P Ca = 1/3000) and all of these are impermeable to anions (negatively charged ions like Cl − , attracted to an “anode”).
Because ions are charged, net ionic flux through an open channel is determined by both the concentration and the electrical gradients across the membrane (electrodiffusion). As discussed above, the potential at which the passive flux of ions resulting from the chemical driving force is exactly balanced by the electrical driving force is called the equilibrium or reversal potential of the channel. In a channel that is perfectly selective for one ion species, the reversal potential equals the thermodynamic equilibrium potential of that ion, E S , which is given by an equation of the form:
where [S i ] and [S o ] are the intracellular and extracellular concentrations of the permeant ion, respectively, z is the valence of the ion, R is the gas constant, F is the Faraday constant, T is the absolute temperature (kelvin), and ln is the natural logarithm (the Nernst equation is this equation applied to K + ). If the current through an open channel is carried by more than one permeant ion, the reversal potential becomes a weighted mean of all the equilibrium potentials.
The activation of voltage-dependent cardiac channels generally increases with membrane depolarization. Channels do not have a sharp voltage threshold for opening. Rather, the dependence of channel activation on membrane potential is a continuous function of voltage and follows a sigmoidal curve ( Fig. 62.3A , blue curve ). The potential at which activation is half-maximal and the steepness of the activation curve govern channel permeability changes in response to changes in membrane potential. Some channels (like Na + , L-type Ca 2+ and transient-outward K + channels) also show inactivation, with a voltage dependence qualitatively similar to activation but generally occurring at more negative voltages ( Fig. 62.3A , gold curve ). Both channel activation and inactivation are time-dependent, with a change in voltage causing a progressive change in gating toward the steady-state values shown in Figure 62.3A . The time constant of a gating process indicates the time required to get to about 63% of steady state, and ranges from about 1 msec (msec, 1/1000 second) for Na + channel activation to about 5 to 10 msec for L-type Ca 2+ channels to 100 msec for delayed-rectifier K + channels. Inactivation is generally about an order of magnitude slower than activation.
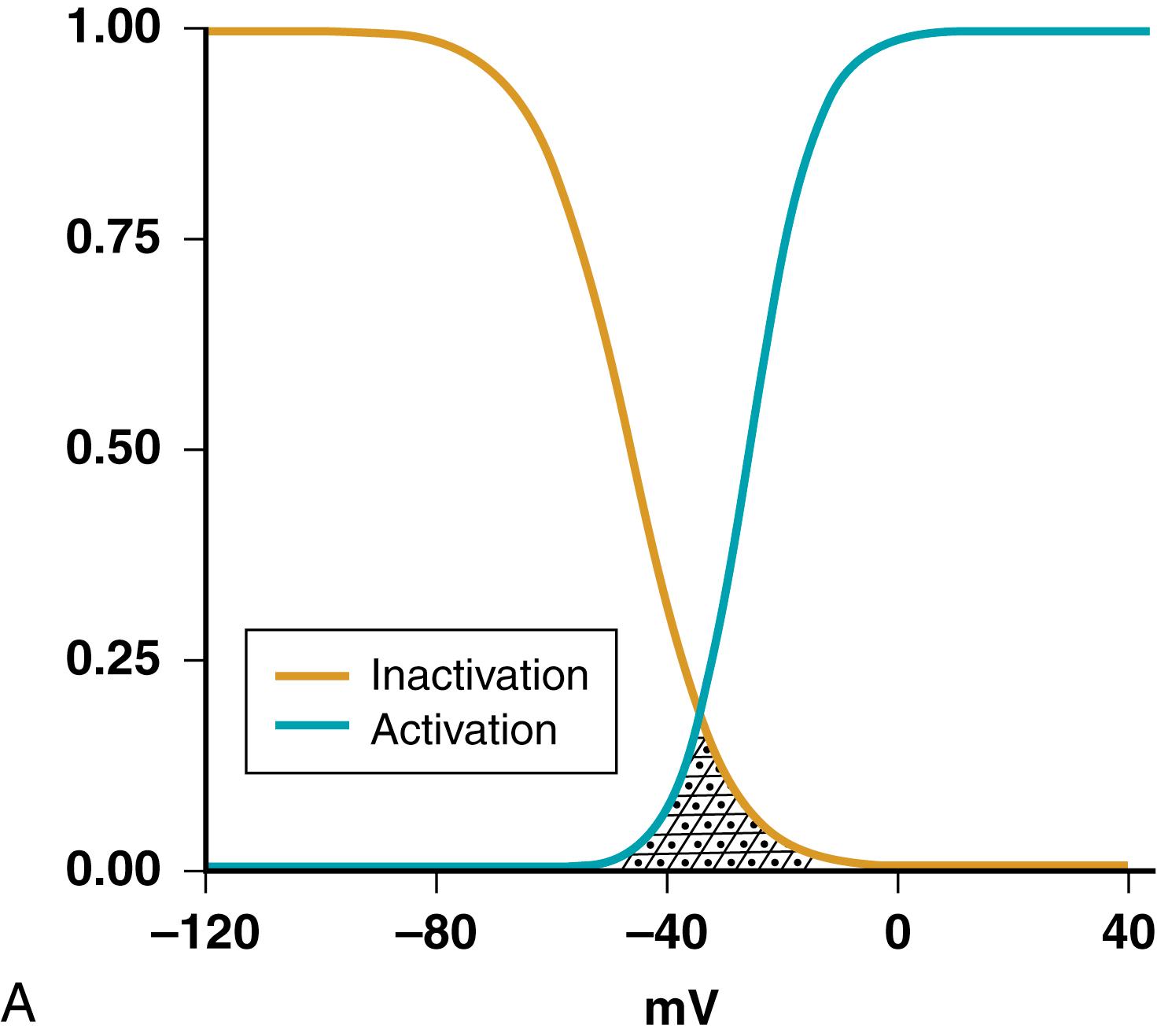
Activation occurs rapidly on membrane depolarization, causing channel opening. Inactivation proceeds with a slight delay because of its slower kinetics, allowing for a substantial number of channels to open and carry current before inactivation occurs. If membrane depolarization persists, the channel remains inactivated and cannot reopen. Inactivation curves of the various cardiac voltage-gated ion channels differ in their voltage dependence. For example, sustained membrane depolarization to −50 mV (as may occur in acutely ischemic myocardium) causes almost complete inactivation of the Na + channel, whereas the L-type Ca 2+ channel (see Voltage-Gated Ca 2+ Channels) exhibits little inactivation at this membrane potential. Activation and inactivation curves can overlap over a voltage range with finite steady-state conductance (hatched area in Fig. 62.3A ), in which case a steady-state noninactivating inward current can flow, potentially causing spontaneous depolarization. L-type Ca 2+ channel and Na + channel window currents have been implicated in the genesis of triggered activity arising from early afterdepolarizations (EADs).
Channels recover from inactivation and then enter the closed state, from which they can be reactivated and open again ( Fig. 62.3B ). Rates of recovery from inactivation vary among the different types of voltage-dependent channels and usually follow monoexponential or multiexponential time courses, with time constants ranging from a few milliseconds for Na + current to several seconds for some subtypes of K + currents.
The whole-cell current amplitude I is the product of the number of functional channels in the membrane available for opening ( N ), the probability that a channel will open ( P o ), and the single-channel current amplitude (i) ( Fig. 62.3C ), or I = N • P o • i . Changes in total current therefore result from alterations in N , P o , i , or any combination of these factors. Changes in the number of available channels in the cell membrane result from up- or down-regulation of the expression of ion channel–encoding genes. The magnitude of the single-channel current amplitude depends in part on the ionic concentration gradient across the membrane. Changes in channel activation (i.e., P o ) can result from phosphorylation or dephosphorylation of the channel protein, or from gene mutations that alter channel gating or conductivity properties.
| Current | Subunit | Functional Properties |
|---|---|---|
| A. Sodium Currents | ||
| I Na | Nav1.5, Nav1.1, Nav1.3, Nav1.6, Nav1.8 (alpha subunits) | TTX-resistant (Nav1.5, Nav1.8) and TTX-sensitive (Nav1.1, Nav1.3, Nav1.6) voltage-gated currents; Nav1.5 is the major cardiac isoform; neuronal Na + channel isoforms contribute to SA node pacemaking and ventricular repolarization |
| B. Calcium Currents | ||
| I Ca.L | Cav1.2 (alpha subunit) | L-type ( l ong lasting, l arge conductance) Ca 2+ currents through voltage-gated Ca 2+ (Cav) channels blocked by dihydropyridine antagonists (e.g., nifedipine), phenylalkylamines (e.g., verapamil), benzodiazepines (e.g., diltiazem), and various divalent ions (e.g., Cd 2+ ); activated by dihydropyridine agonists (e.g., Bay K 8644); responsible for phase 0 depolarization and propagation in SA and AV nodal tissue and contributing to the plateau of atrial, His-Purkinje, and ventricular cells; main trigger of Ca 2+ release from the sarcoplasmic reticulum (Ca 2+ -induced Ca 2+ release); the noninactivating or “window” component underlies EADs |
| I Ca.T | Cav3.1/alpha 1G (alpha subunit) | T-type ( t ransient current, t iny conductance) Ca 2+ currents through Cav channels blocked by mibefradil and efonidipine but insensitive to dihydropyridines; may contribute an inward current to the later phase of phase 4 depolarization in pacemaker cells and action potential propagation in AV nodal cells; role in triggering Ca 2+ -induced Ca 2+ release uncertain |
| I f | HCN4 (alpha subunit) | Hyperpolarization-activated “funny” current carried by Na + and K + in SA and AV nodal cells and His-Purkinje cells; involved in generating phase 4 depolarization; increases the rate of impulse initiation in pacemaker cells |
| C. Potassium Currents | ||
| I K1 | Kir2.1 (alpha subunit) | K + current through inwardly rectifying K + (Kir) channels, voltage-dependent block by Ba 2+ at micromolar concentrations; responsible for maintaining resting the membrane potential in atrial, His-Purkinje, and ventricular cells; channel activity is a function of both membrane potential and [K + ] o ; inward rectification appears to result from depolarization-induced internal block by Mg 2+ and neutral or positively charged amino acid residues in the cytoplasmic channel pore |
| I K.G (I K.ACh , I K.Ade ) | Kir3.1/Kir3.4 (alpha subunit) | Inwardly rectifying K + current activated by muscarinic (M 2 ) and purinergic (type 1) receptor stimulation via GTP regulatory (G) protein signal transduction; expressed in SA and AV nodal cells and atrial cells, where it causes hyperpolarization and action potential shortening; activation causes negative chronotropic and dromotropic effects |
| I Ks | KvLQT1, Kv7.1 (alpha subunit)/minK (beta subunit) | K + current carried by a voltage-gated K + (Kv) channel (delayed rectifier K + channel); plays a major role in determining phase 3 of the action potential |
| I Kr | hERG, Kv11.1 (alpha subunit)/MiRP1 (beta subunit) | Rapidly activating component of delayed rectifier K + current; I Kr specifically blocked by dofetilide and sotalol in a reverse use–dependent manner; inward rectification of I Kr results from depolarization-induced fast inactivation; plays a major role in determining the APD |
| I Kur | Kv1.5 (alpha subunit) | K + current through a Kv channel with ultrarapid activation but ultraslow inactivation kinetics; expressed in atrial myocytes; determines the APD |
| I K.Ca | SK1-3 (alpha subunit) | K + current through small-conductance Ca 2+ -activated channels; blocked by apamin and expressed in human atrial and ventricular myocytes; determines the APD; upregulated in failing cardiomyocytes |
| I to (I to1 , I A ) | Kv4.3 (alpha subunit)/KChIP2 (beta subunit) | Transient outward K + current through voltage-gated (Kv) channels; exhibits fast activation and inactivation and recovery kinetics; blocked by 4-aminopyridine in a reverse use–dependent manner; contributes to the time course of phase 1 repolarization; transmural differences in I to properties contribute to regional differences in early repolarization |
| D. Chloride Currents | ||
| I Cl.Ca (I to2 ) | ? | 4-Aminopyridine–resistant transient outward current carried by Cl − ions; activated by an increase in intracellular calcium level; blocked by stilbene derivatives (SITS, DIDS); contributes to the time course of phase 1 repolarization; may underlie spontaneous transient inward currents under conditions of Ca 2+ overload; molecular correlate uncertain |
| I Cl.cAMP | ? | Time-independent chloride current regulated by the cAMP/adenylate cyclase pathway; slightly depolarizes resting membrane potential and significantly shortens the APD; antagonizes action potential prolongation associated with beta-adrenergic stimulation of I Ca.L |
| I Cl.swell or I Cl.vol | ? | Outwardly rectifying, swelling-activated Cl − current; inhibited by 9-anthracene carboxylic acid; activation causes resting membrane depolarization and action potential shortening |
| I K.ATP | Kir6.2 (alpha subunit)/SUR | Time-independent K + current through Kir channels activated by a fall in intracellular ATP concentration; inhibited by sulfonylurea drugs, such as glibenclamide; activated by pinacidil, nicorandil, cromakalim; causes shortening of the APD during myocardial ischemia or hypoxia |
| I Cir.swell | ? | Inwardly rectifying, swelling-activated cation current; permeable to Na + and K + ; inhibited by Gd 3+ ; depolarizes resting membrane potential and prolongs terminal (phase 3) repolarization |
| E. Electrogenic Pumps and Exchangers | ||
| I Na/Ca | NCX1.1 | Current carried by Na + /Ca 2+ exchanger; causes net Na + outward current and Ca 2+ inward current (reverse mode) or net Na + inward and Ca 2+ outward current (3 Na + for 1 Ca 2+ ); direction of Na + flux depends on membrane potential and intracellular and extracellular concentrations of Na + and Ca 2+ ; Ca 2+ influx mediated by I Na/Ca can trigger SR Ca 2+ release; underlies I ti (transient inward current) under conditions of intracellular Ca 2+ overload |
| I Na/K | Alpha subunit/beta subunit | Na + outward current generated by Na + ,K + -ATPase (stoichiometry: 3 Na + leave and 2 K + enter); inhibited by digitalis |
| I ti | ? | Transient inward current activated by Ca 2+ waves; I ti possibly reflects 3 Ca 2+ -dependent components: I NCX , I Cl.Ca , and a TRPM4 (transient receptor potential cation channel, member 4 gene)–mediated current |
| F. Electroneutral Ion-Exchanging Proteins | ||
| Ca 2+ -ATPase | SERCA2 | Extrudes cytosolic calcium |
| Na/H | Cardiac myocytes express isoform NHE1 | Exchanges intracellular H + for extracellular Na + ; specifically inhibited by the benzoylguanidine derivatives HOE 694 and HOE 642; inhibition causes intracellular acidification |
| Cl − -HCO 3 − | Exchanges intracellular HCO 3 − for external Cl − ; inhibited by SITS | |
| Na + -K + -2Cl − | Na-K-Cl NKCC1 |
Cotransporter blocked by amiloride |
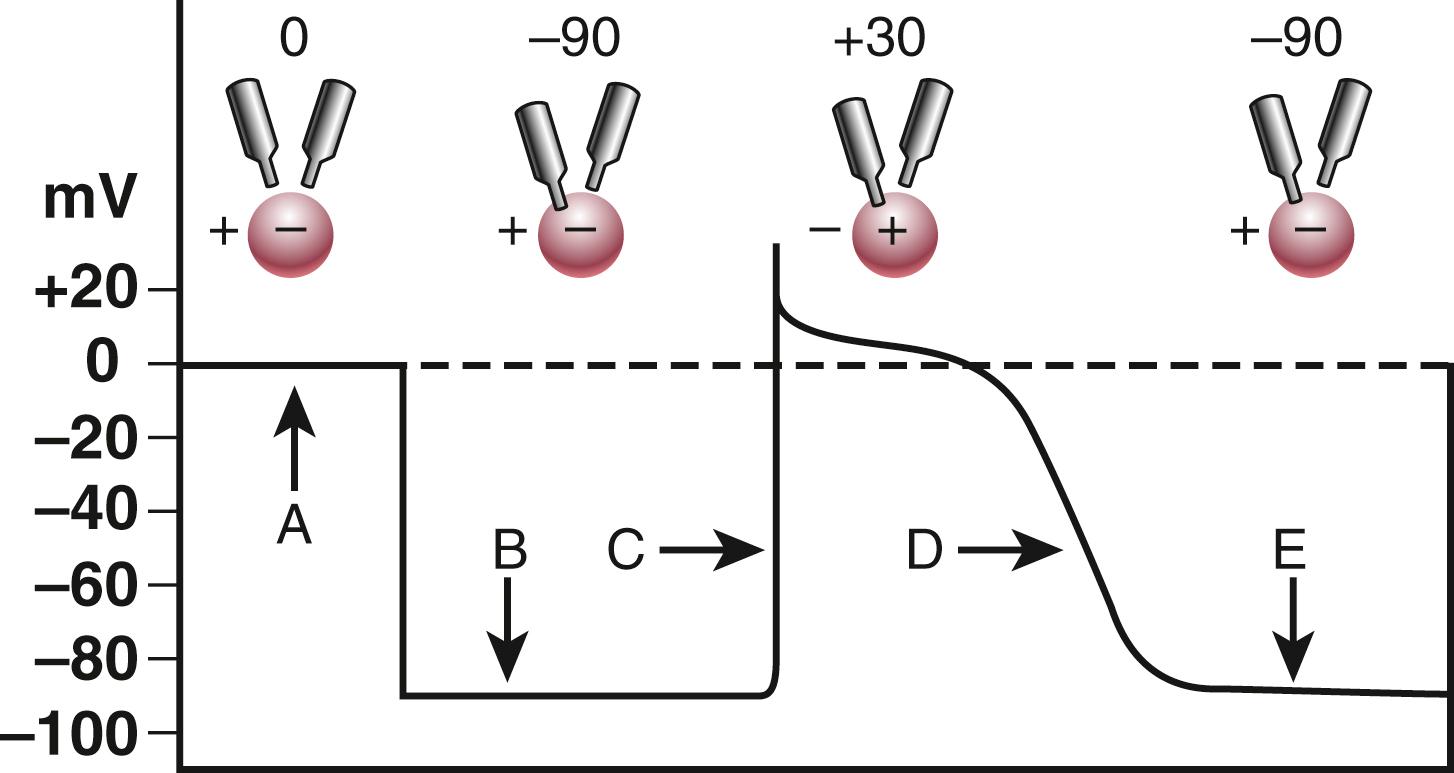
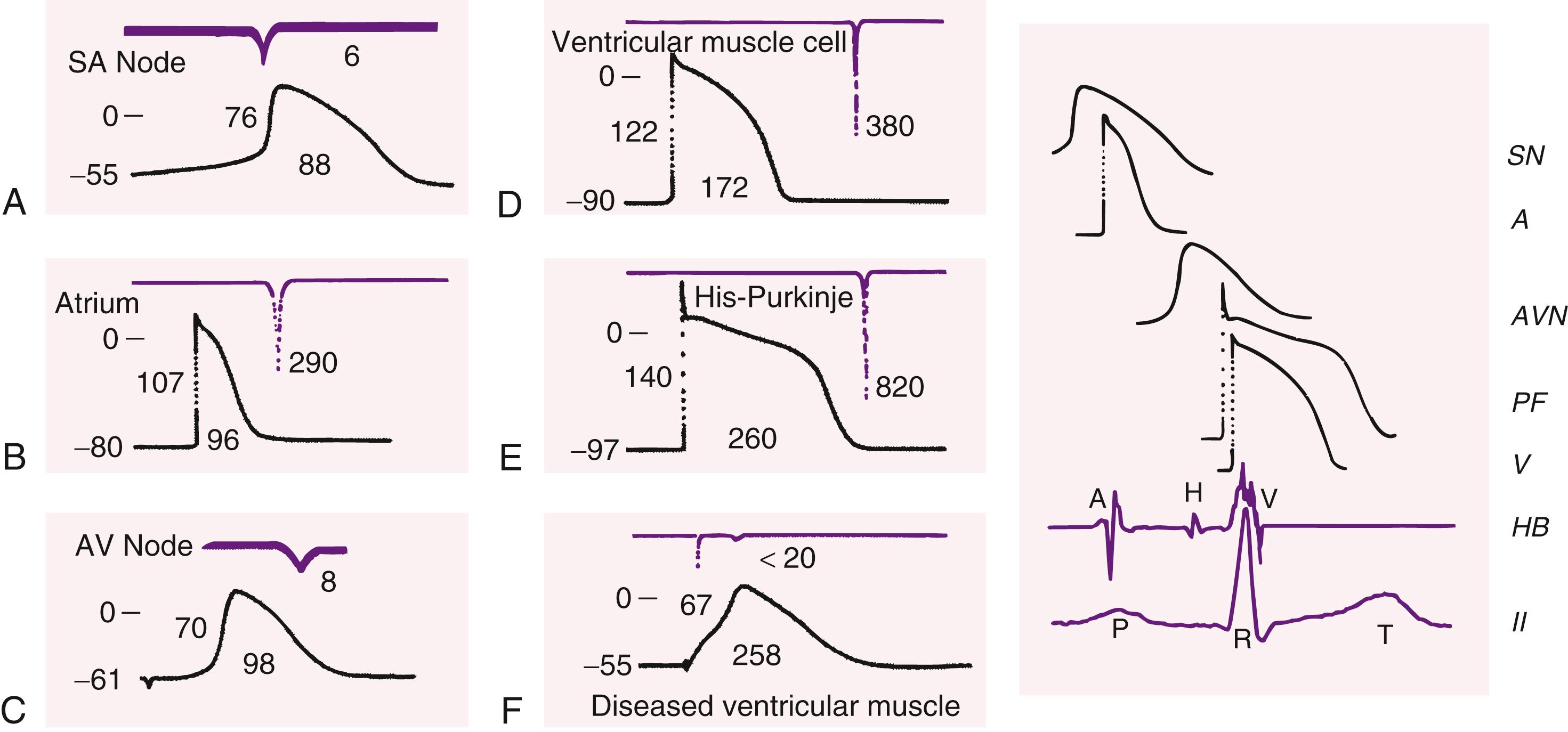
As discussed above, the cardiac AP consists of five phases: phase 0, upstroke or rapid depolarization; phase 1, early rapid repolarization; phase 2, plateau; phase 3, final rapid repolarization; and phase 4, resting membrane potential and diastolic depolarization (see Fig. 62.2 and eFig. 62.1 ). These phases are the result of passive ion fluxes moving down their electrochemical gradients established by active ion pumps and exchange mechanisms. Each ion moves primarily through its own ion-specific channel. Different regions in the heart have different AP morphologies. However, two specific regions have quite distinct APs from the rest of the heart: the SA and AV nodes. Unlike the APs shown in Figure 62.2 , these areas have APs with much less negative resting potentials and much slower phase-0 upstrokes ( Fig. 62.4 ), often referred to as “slow-response APs.” The following discussion explains the detailed electrogenesis of each of these phases and how it can be altered.
The intracellular potential during electrical quiescence in diastole is −50 to −95 mV, depending on the type of cell ( Table 62.2 and Figure 62.4 ).
| Property | Sa Nodal Cell | Atrial Myocyte | Av Nodal Cell | His Purkinje Cell | Ventricular Myocyte |
|---|---|---|---|---|---|
| Resting potential (mV) | −50 to −60 | −80 to −90 | −60 to −70 | −90 to −95 | −80 to −90 |
| Action Potential Features | |||||
| Amplitude (mV) | 60–70 | 110–120 | 70–80 | 120 | 110–120 |
| Overshoot (mV) | 0–10 | 30 | 5–15 | 30 | 30 |
| Duration (msec) | 100–300 | 100–300 | 100–300 | 300–500 | 200–300 |
| V ̇max (V/sec) | 1–10 | 100–200 | 5–15 | 500–700 | 100–200 |
| Propagation velocity (m/sec) | <0.05 | 0.3–0.4 | 0.1 | 2–3 | 0.3–0.4 |
| Fiber diameter (μm) | 5–10 | 10–15 | 1–10 | 100 | 10–15 |
Outward potassium current through open, inwardly rectifying K + channels (I K1 ) under normal conditions determines the resting membrane potential in atrial and ventricular myocytes, as well as in the specialized conducting cells of the His-Purkinje system. Slow-response tissues have a less negative resting potential because they have much lower I K1 channel-density in their membranes than other parts of the heart.
The intracellular ion concentrations that govern the AP are set by a group of ion pumps and exchangers that transport ions more slowly than open ion channels, but are active continuously throughout the cardiac cycle. The most important pump in establishing the transmembrane ionic distribution gradient is the Na-K pump, which pumps Na + out of the cell and simultaneously pumps K + into the cell against their respective chemical gradients, keeping the intracellular K + concentration high and the intracellular Na + concentration low. The Na-K pump is “electrogenic”: each cycle of the pump carries 3 Na + ions out for every 2 K + ions pumped in, leaving a net negative intracellular charge. The rate of Na + -K + pumping must increase as the heart rate increases to maintain the same ionic gradients, because the cell gains a small amount of Na + and loses a small amount of K + with each depolarization. Rapid cardiac activation makes the resting potential more negative because of the electrogenic properties of the activated Na-K pump. The energy for the Na-K pump is generated by its catalytic activity that breaks high-energy phosphate bonds in adenosine triphosphate (ATP), so it is often called the “Na + ,K + -ATPase.” Excess inhibition of Na + ,K + -ATPase function (e.g., by digitalis glycoside toxicity) can cause substantial cardiac-cell depolarization and seriously impair cardiac electrical function, leading to conduction block and arrhythmias.
The resting potential can be made more negative by interventions that increase the K + permeability; for example, both adenosine and acetylcholine (ACh) activate a G-protein coupled K + channel (I K.G , Table 62.1 ) and increase resting K + conductance. In fast-response tissues, the normal resting K + conductance is high and I K.G activation produces small changes in resting potential. However, in SA and AV nodes, with very limited baseline K + conductance, I K.G activation substantially increases resting K + conductance and can have a major hyperpolarizing effect that slows automaticity and conduction.
Cardiac activation normally proceeds from the SA node pacemaker and is conducted throughout the heart. Electrical changes in the AP follow a relatively fixed time and voltage relationship that differs according to specific cell types ( Fig. 62.5 ). The AP can also be initiated by an electrical stimulus, as is induced by an artificial pacemaker. Normally, the AP morphology is independent of the size of the depolarizing stimulus, provided that the pulse exceeds a threshold value. Small, subthreshold depolarizing stimuli depolarize the membrane in proportion to the strength of the stimulus, but fail to elicit an AP. When the stimulus is sufficiently intense to depolarize membrane potential to a threshold value in the range of about −70 to −65 mV (for normal fast-response tissues), an “all-or-none” response results.
The upstroke of the cardiac AP in fast-response tissues results from a rapid increase in membrane conductance for Na + . Rapid depolarization opens Na + channels before they have a chance to close via inactivation. When the depolarization is sufficiently rapid, Na + channel opening allows Na + ions to enter the cell, depolarizing it rapidly and causing more channels to open, producing further depolarization in a self-sustaining process (the “all-or-none” phase 0 depolarization). This process stops when Na + channels inactivate to a significant degree (within 1 to 2 msec), allowing the membrane to reach a voltage of about +40 mV, near the Na + equilibrium potential (+60 mV).
The rate at which depolarization occurs during phase 0, that is, the maximum rate of change in voltage over time, is indicated by the expression dV/dt max or V ̇max (see Table 62.2 ). V ̇max (dV/dt max ) is an indicator of the rate and magnitude of phase 0 Na + entry into the cell, which is a determinant of tissue conduction velocity (CV). Depolarization must be rapid in order to initiate a fast-response AP. A slow depolarization allows Na + channels to inactivate, reducing the maximal conductance they can achieve when activated from that depolarized potential. Thus, clinical processes like hyperkalemia, which slowly depolarize the cell (by reducing the transmembrane K + concentration gradient and consequently the K + equilibrium potential) result in reduced phase 0 Na + current and can produce important conduction slowing.
In cardiac Purkinje fibers, the SA node region, and to a lesser extent, ventricular muscle, different populations of Na + channels exist. By far the most common is the tetrodotoxin (TTX)-resistant Nav1.5 isoform (encoded by the SCN5A gene). The Nav1.8 TTX-resistant isoform ( SCN10a ) contributes little to peak I Na , but plays an important role in the late Na + current (I NaL ) that contributes importantly to the prolongation of AP-duration (APD) with cardiac disease or ion-channel mutations. TTX-sensitive Na + channels may also participate in I NaL , although the precise contribution remains to be better defined.
Normal fast-response APs have phase 0 dV/dt max values of the order of 200 to 1000 mV/msec. Slow-response APs in the SA and AV nodes have much slower upstrokes (of the order of 4 to 20 mV/msec) because of the slower activation of Ca 2+ channels compared to Na + channels (see Figs. 62.4 and 62.5 ). The slow phase 0 upstroke is associated with much slower conduction in slow versus fast response tissues. Slow conduction through the AV node is responsible for creating a delay between atrial and ventricular activation, helping to ensure optimal efficiency of the atrial “primer pump” function to optimize ventricular filling just before ventricular contraction. Under certain circumstances, diseased working myocardial cells can have slow-response type cells (see Fig. 62.5F ).
The threshold for activation of I Ca.L is about −30 to −40 mV. In fibers of the fast-response type, I Ca.L is normally activated during phase 0 caused by the fast I Na . Current flows through both fast and slow channels during the latter part of the AP upstroke. However, I Ca.L is much smaller than the peak I Na and therefore contributes little to the AP until the fast I Na is inactivated after completion of phase 0. Thus, I Ca.L affects mainly the plateau of APs recorded in atrial and ventricular muscle and His-Purkinje fibers. I Ca.L may play a prominent role in depolarized fast-response cells in which I Na has been inactivated, if conditions are appropriate for slow-channel activation. Although T-type Cav channels have not been directly recorded in human myocardium, the corresponding genes have been cloned from human hearts, and experimental evidence in animals has suggested that these channels might play an important role in determining SA node automaticity and AV nodal conduction.
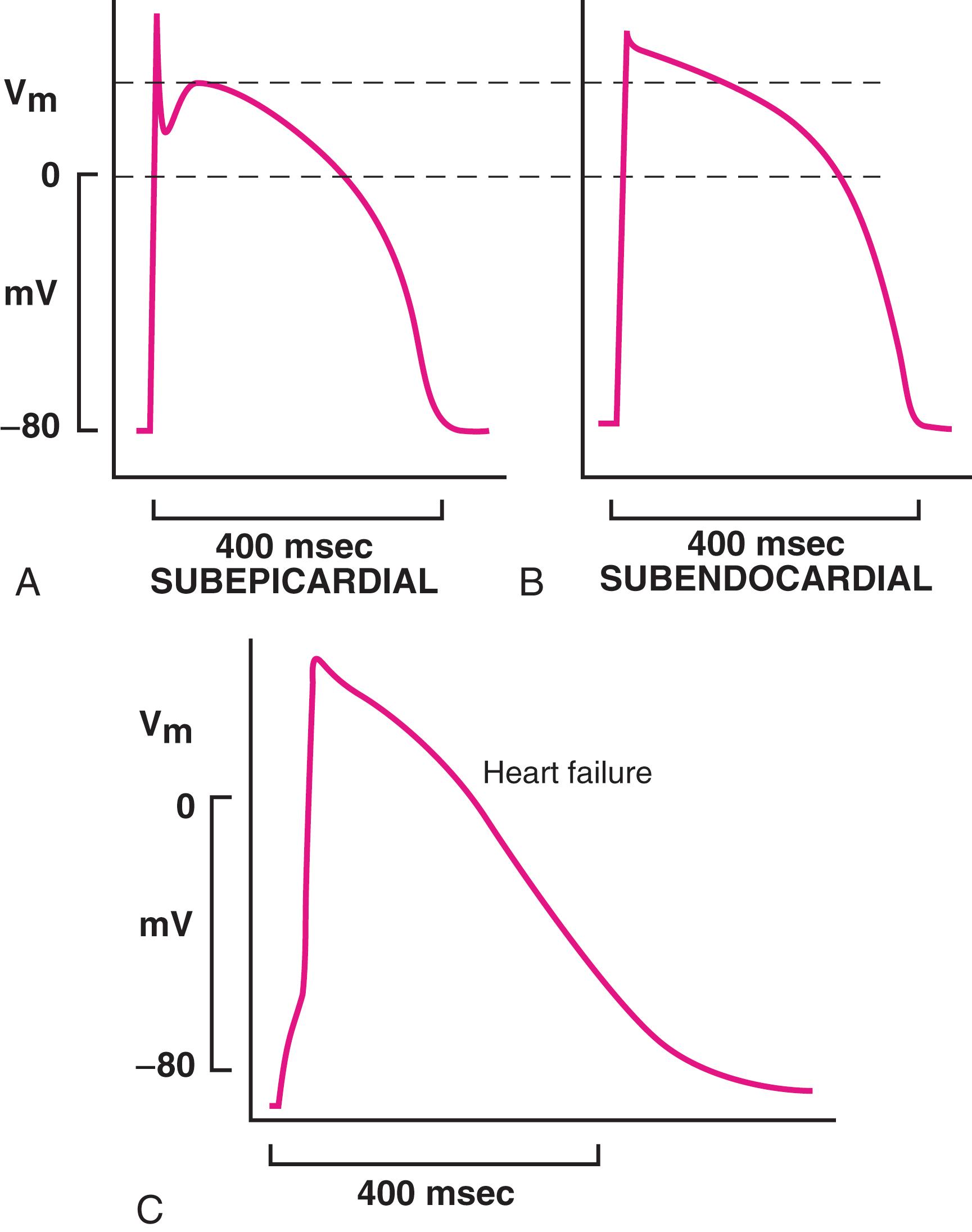
Following phase 0, the membrane repolarizes rapidly and transiently to almost 0 mV (early notch), partly because of inactivation of I Na and concomitant activation of several outward currents. The most important of these in the human heart is the 4-aminopyridine–sensitive transient outward K + current, commonly termed I to (or I to1 ), which is activated rapidly by depolarization and then rapidly inactivates. Both the density and the recovery of I to from inactivation exhibit transmural gradients in the left and right ventricular (RV) free wall, with the density decreasing and reactivation becoming progressively prolonged from epicardium to endocardium. Transmural differences in the expression of KChIP2, the auxiliary subunit that forms the I to channel in working atrial and ventricular muscle along with Kv4.3 pore-forming alpha subunits, contributes to the transmural gradient in I to properties and densities. This gradient gives rise to regional differences in AP shape, with increasingly slower phase 1 restitution kinetics and diminution of the notch along the transmural axis ( eFig. 62.2 ). These regional differences create transmural voltage gradients, thereby increasing dispersion of repolarization and allowing for arrhythmogenic transmural current flow, contributing to arrhythmogenesis in Brugada syndrome (see Chapter 63, Chapter 67 ). Downregulation of I to is at least partially responsible for slowing of phase 1 repolarization in failing human cardiomyocytes.
Studies have demonstrated that these changes in the phase 1 notch of the cardiac AP cause a reduction in the kinetics and peak amplitude of the AP–evoked intracellular Ca 2+ transient because of failed recruitment and synchronization of SR Ca 2+ release through I Ca.L ( eFig. 62.3 ). Thus, modulation of I to appears to play a physiologic role in controlling cardiac excitation-contraction coupling. It remains to be determined whether transmural differences in phase 1 repolarization translate into similar differences in regional contractility that are important for overall contractile function.
The 4-aminopyridine–resistant, Ca 2+ -activated chloride current I Cl.Ca (or I to2 ) might also contribute to outward current during phase 1 repolarization. This current is activated by the AP–evoked intracellular Ca 2+ transient. Other chloride currents may also play a role in early repolarization, such as the cyclic adenosine monophosphate (cAMP)- or swelling-activated chloride conductances I Cl.cAMP and I Cl.swell .
During the plateau phase, which may last several hundred milliseconds, membrane conductance of all ions falls to rather low values. Accordingly, smaller changes in current are needed to produce changes in transmembrane potential than near the resting potential. This phenomenon makes the plateau a vulnerable time for the generation of arrhythmogenic afterdepolarizations (see discussion of EADs below). The plateau is maintained by competition between the outward current carried by K + and the inward current carried by Ca 2+ moving through I Ca,L and Na + being exchanged for internal Ca 2+ by the NCX. After depolarization, I K1 conductance falls to low levels during the plateau as a result of inward rectification, despite the large electrochemical driving force on K + ions.
The plateau is a critical time for Ca 2+ entry through activated L-type Ca 2+ channels. The entry of Ca 2+ , principally during the plateau, triggers a much larger secondary release of Ca 2+ from SR stores and is an essential component of cardiac excitation-contraction coupling (see Chapter 46 ).
Several potassium currents are open during the plateau phase, including the rapid (I Kr ) and slow (I Ks ) components of the delayed rectifier current I K (see Voltage-Gated K + Channels). “Delayed rectification” refers to the time-dependent opening of the I K channel. In addition, I Kr shows a phenomenon called “inward rectification,” which involves a decrease in channel opening at more positive potentials. Inward rectification is a prominent feature of the background I K1 channel, whose conductance drops dramatically at positive potentials to prevent wasteful excess loss of K + and allow efficient phase 0 depolarization. I Kr also shows prominent inward rectification, due to extremely rapid inactivation that occurs during depolarization. This fast inactivation mechanism is sensitive to changes in extracellular K + , with the degree of inactivation being accentuated at low extracellular K + concentrations, explaining how hypokalemia can prolong APD (by decreasing I Kr ).
I Ks also contributes to plateau duration. Mutations in the KvLQT1 subunit, which in combination with the I Ks ancillary (beta) subunit ( KCNE1, encoding a protein also called minK) carries I Ks , are associated with abnormally prolonged ventricular repolarization (long-QT syndrome [LQTS] type 1; see Chapter 63, Chapter 67 ). Although I Ks activates slowly compared to the APD, it is only slowly inactivated. Therefore, increases in heart rate cause I Ks activation to accumulate, contributing to APD abbreviation at higher heart rates.
In conditions of reduced intracellular ATP concentration (e.g., hypoxia, acute ischemia), K + efflux through activated I KATP channels reduces APD and increases K + concentration in the extracellular space, which in turn decreases the K driving force, I K1 amplitude, and cell resting potential. Other ionic mechanisms that control plateau duration include I CaL inactivation and steady-state (window-current) components of both I Na and I Ca.L .
Repolarization of the terminal portion of the AP proceeds rapidly largely because of changes in two currents: time-dependent inactivation of I CaL , with a decrease in the inward movement of positive charges, and activation of repolarizing K + currents, particularly I Kr and I Ks , which increase the movement of positive charges out of the cell. As the cell repolarizes, the transmembrane potential moves to voltages at which the inward rectification of I K1 is removed, so I K1 contributes substantially to the terminal part of phase 4. A small-conductance Ca 2+ -activated K + current, I KCa , expressed in human atrial myocytes, may also contribute to phase 3 repolarization.
Loss-of-function mutations in the human ether-a-go-go–related or hERG gene (KCNH2) , which encodes the pore-forming (α) subunit of I Kr , delay phase 3 repolarization and predispose to the development arrhythmias associated with LQTS. A wide range of non-cardiac drugs, such as erythromycin, many antipsychotics, antimalarial/anti-inflammatory drugs like hydroxychloroquine, and antifungal drugs such as ketoconazole, inhibit I Kr and can cause acquired forms of LQTS (see Chapter 63, Chapter 67 ). A decrease in I K1 function, as occurs in heart failure or mutations in the associated KCNJ2 gene, prolong APD and reduce resting membrane potential.
Normally, the membrane potential of atrial and ventricular muscle cells remains steady throughout diastole. In specialized conducting cells of the His-Purkinje system and in the SA and AV nodes, the resting membrane potential does not remain constant in diastole but gradually depolarizes (see Figs. 62.4 and 62.5 ). The property possessed by spontaneously automatic cells is called spontaneous phase 4 diastolic depolarization. When spontaneous phase 4 depolarization reaches a voltage at which a sufficient number of phase 0 inward current channels (Na + channels in His-Purkinje system, Ca 2+ channels in SA and AV nodes) open, spontaneous cell-firing occurs. The voltage at which an AP is generated is called the “threshold potential.” The time from the maximum (most negative) diastolic potential (MDP) at the end of phase 3 to the arrival at threshold potential determines the “intrinsic” spontaneous cycle length of the tissue. The intrinsic rate of SA node automaticity normally exceeds the intrinsic rate of other potentially automatic pacemaker sites; thus the SA mode maintains dominance of the cardiac rhythm. The SA node is heavily innervated by the parasympathetic and sympathetic nervous systems. Parasympathetic activation slows SA node automaticity and sympathetic activation accelerates it. This dual control allows for very fine regulation of SA node rate in relation to the body’s needs, with heart rate being the most important single determinant of cardiac output (see Fig. 62.1 ). Enhanced or abnormal automaticity at other sites (as well as other arrhythmia mechanisms, see below) can cause them to discharge at rates faster than the SA node and usurp control of cardiac rhythm for one or many cycles (see Chapter 65 ). The intrinsic pacemaking ability of other tissues produces important “escape” rhythms should the sinus node fail or should conduction between the atria and ventricles be blocked because of disease in the AV node or specialized His-Purkinje ventricular conducting system.
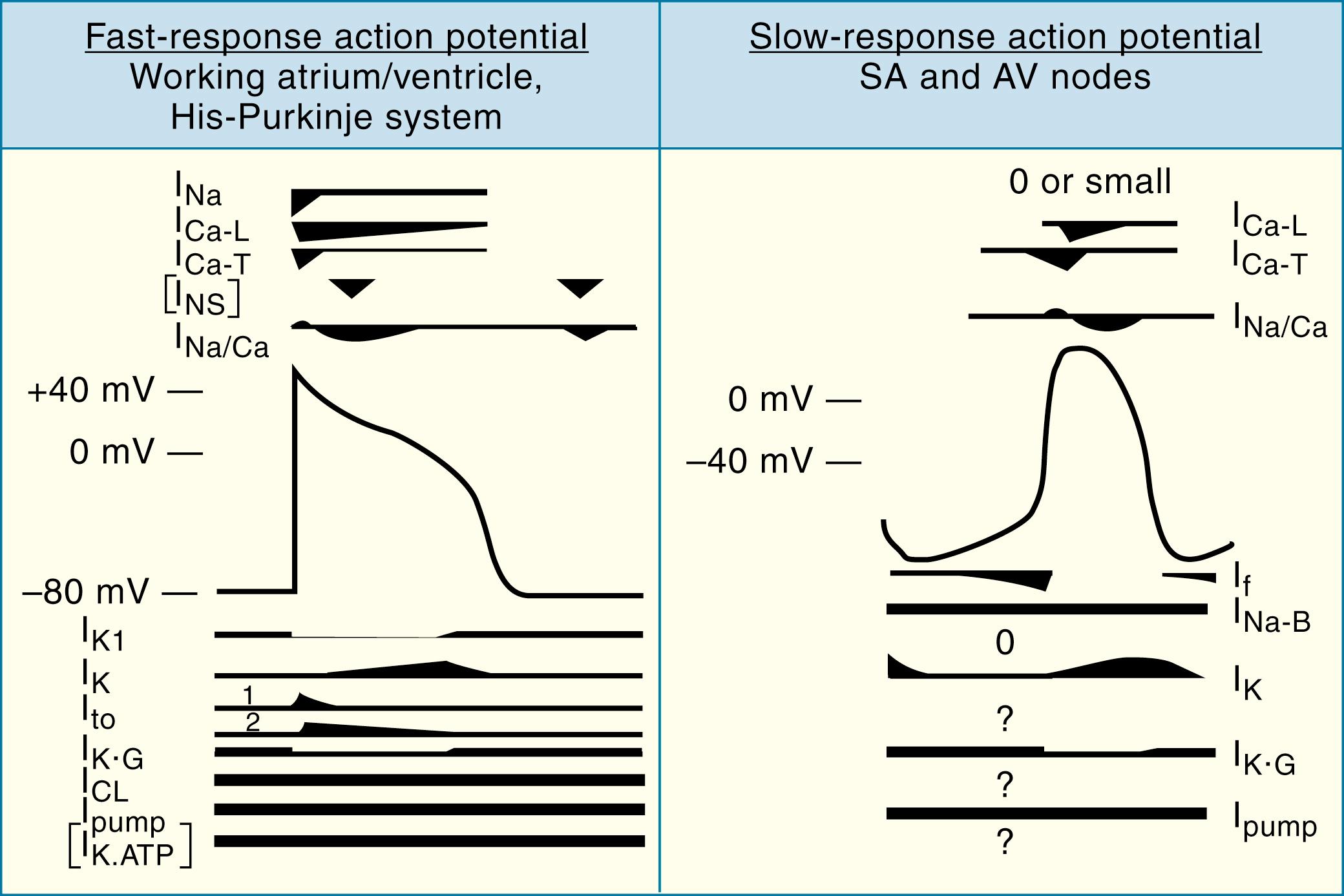
Two models of contributors to SA node pacemaking have been proposed. In the “membrane clock” model, HCN channels (see Cardiac Pacemaker Channels and Table 62.1 ) are activated by repolarization from the plateau to normal diastolic membrane potentials. HCN channels carry a current called I f (“funny current”), often called the “pacemaker current,” with the unusual feature of time-dependent activation at negative potentials (unlike all other voltage-dependent cardiac channels). During the diastolic interval between consecutive APs, the probability of HCN channels being open increases. Open HCN channels conduct both Na + and K + , but at these negative membrane potentials at which the driving force for Na + is high and for K + is low, Na + entry predominates. The inward Na + current through HCN channels depolarizes pacemaker cells to threshold, repetitively triggering APs to generate a periodically firing pacemaker. ,
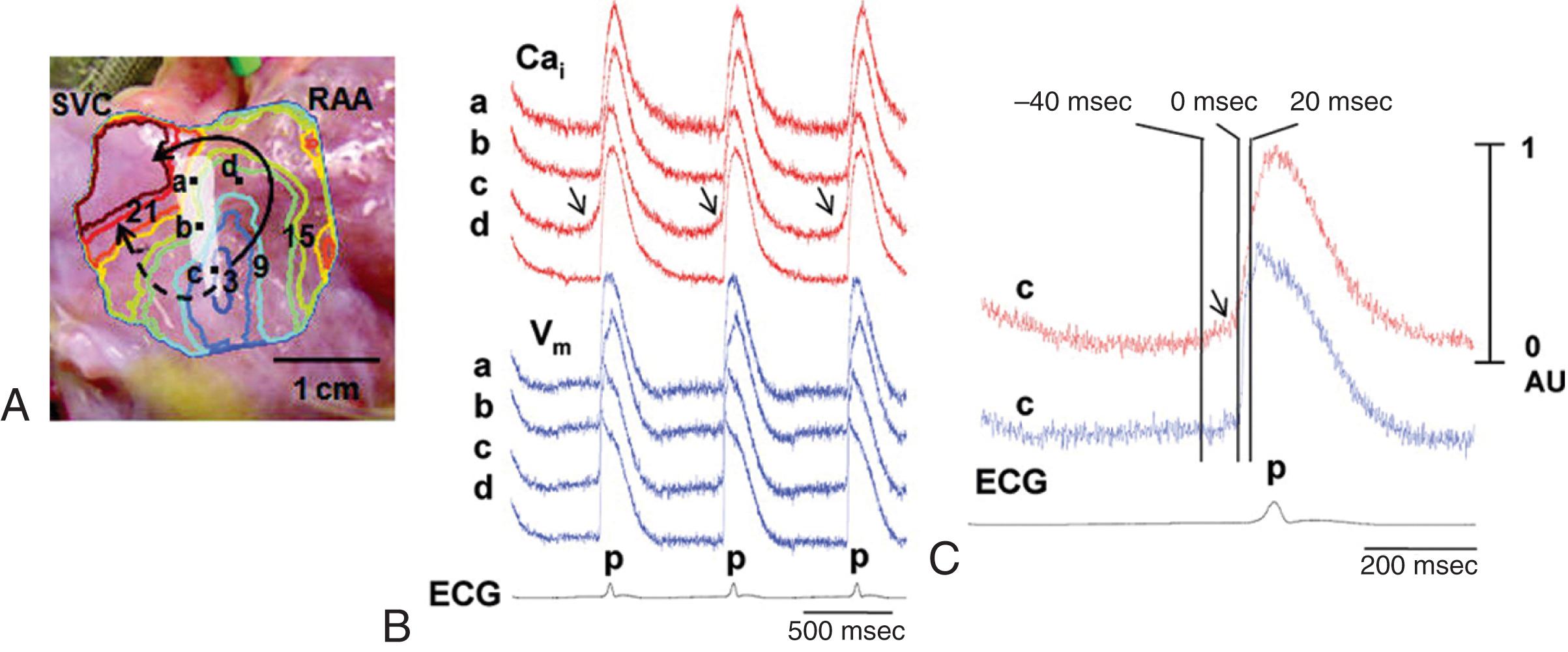
In the “Ca 2+ clock” model, periodic increases in [Ca 2+ ] i serve as an internal generator of rhythmic signals that are transformed into changes in membrane voltage via modulation of calcium-sensitive ion channels and transporters in the cell membrane. This concept is illustrated in Figure 62.6 , in which simultaneous [Ca 2+ ] i and voltage measurements in isolated SA node myocytes are used as an example. Local submembrane increases in [Ca 2+ ] i (denoted by the white arrows in Fig. 62.6B ) occurring during the latter part of the spontaneous diastolic depolarization (transmembrane APs are shown in blue) precede the rapid upstroke of the AP. SR Ca 2+ -release activates the Na + /Ca 2+ exchange inward (i.e., depolarizing) current (I NCX ), which then results in membrane depolarization, which activates membrane L-type Ca 2+ channels to initiate the AP phase-0 upstroke. Thus, the NCX plays an essential role in converting the driver intracellular Ca 2+ signals into membrane (i.e., voltage) signals. Once an AP has been initiated, two highly interacting, concurrent series of events proceed ( Fig. 62.6C ). In a surface membrane delimited series of events, depolarization-induced activation of I K leads to membrane repolarization, which is followed by slow diastolic depolarization via a number of inward currents, particularly I f and I CaT (see Table 62.1 ). In a second, parallel cycle of events, AP–induced SR Ca 2+ release is followed by Ca 2+ reuptake into the SR, giving rise to spontaneous Ca 2+ -release and subsequent inward I NCX .
![FIGURE 62.6, A, Sympathetic stimulation of heart rate in the sinoatrial node (SAN). Simulated SAN action potentials during baseline (NSR) and sympathetic stimulation (ST [sinus tachycardia]) . Sympathetic stimulation increases the rate of diastolic depolarization (not shown) and shifts the maximum diastolic potential to a less negative value, thereby accelerating action potential firing. B and C, Spontaneous sarcoplasmic reticulum (SR) Ca 2+ -release events trigger membrane excitation in SAN myocytes. B, Confocal line scan images of Ca 2+ signals measured in spontaneously beating rabbit sinoatrial node cells (SANC) with simultaneous recording (blue lines) of transmembrane action potentials; the orientation of the scan line is shown in the inset. Arrows in the confocal image show the local Ca 2+ release in the submembrane space during late diastolic depolarization that precedes the rapid upstroke of the action potential. C, Model of sinoatrial node cell pacemaking, as suggested by Maltsev and coworkers. I NCX , Na + /Ca 2+ exchange current; DD, diastolic depolarization; LCR, local Ca 2+ release; SERCA, sarcoendoplasmic reticulum Ca 2+ -ATPase. FIGURE 62.6, A, Sympathetic stimulation of heart rate in the sinoatrial node (SAN). Simulated SAN action potentials during baseline (NSR) and sympathetic stimulation (ST [sinus tachycardia]) . Sympathetic stimulation increases the rate of diastolic depolarization (not shown) and shifts the maximum diastolic potential to a less negative value, thereby accelerating action potential firing. B and C, Spontaneous sarcoplasmic reticulum (SR) Ca 2+ -release events trigger membrane excitation in SAN myocytes. B, Confocal line scan images of Ca 2+ signals measured in spontaneously beating rabbit sinoatrial node cells (SANC) with simultaneous recording (blue lines) of transmembrane action potentials; the orientation of the scan line is shown in the inset. Arrows in the confocal image show the local Ca 2+ release in the submembrane space during late diastolic depolarization that precedes the rapid upstroke of the action potential. C, Model of sinoatrial node cell pacemaking, as suggested by Maltsev and coworkers. I NCX , Na + /Ca 2+ exchange current; DD, diastolic depolarization; LCR, local Ca 2+ release; SERCA, sarcoendoplasmic reticulum Ca 2+ -ATPase.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/MechanismsofCardiacArrhythmias/8_3s20B9780323722193000621.jpg)
In reality, the calcium and membrane clock systems function together, to ensure that the important SA node pacemaking function is protected by system redundancy, somewhat like dual computers producing a failsafe system controlling key aircraft guidance functions.
The rate of SA node discharge is regulated by autonomic and other influences. Alterations in the slope of diastolic depolarization, MDP, or threshold potential can alter the discharge rate. For example, if the slope of diastolic depolarization steepens, the discharge rate increases (e.g., Fig. 62.6A , dashed line). The molecular mechanisms that mediate acceleration of the SA node discharge rate in response to adrenergic stimulation are complex. Adrenergic stimulation increases inward HCN current, mainly by virtue of cyclic AMP shifting the HCN channel activation curve to more depolarized potentials. In addition, cAMP-activation of protein kinase A (PKA) increases phosphorylation of key Ca 2+ -handling proteins like the cardiac ryanodine-receptor (RyR2), phospholamban (see Chapter 46 ), SERCA, and voltage-gated Ca 2+ channels. Phosphorylation of these proteins increases the rate of spontaneous SR Ca 2+ release and SR Ca 2+ uptake via synergistic activation of these proteins. ACh shifts the MDP to more negative values, moving it further away from threshold and slowing the spontaneous firing rate.
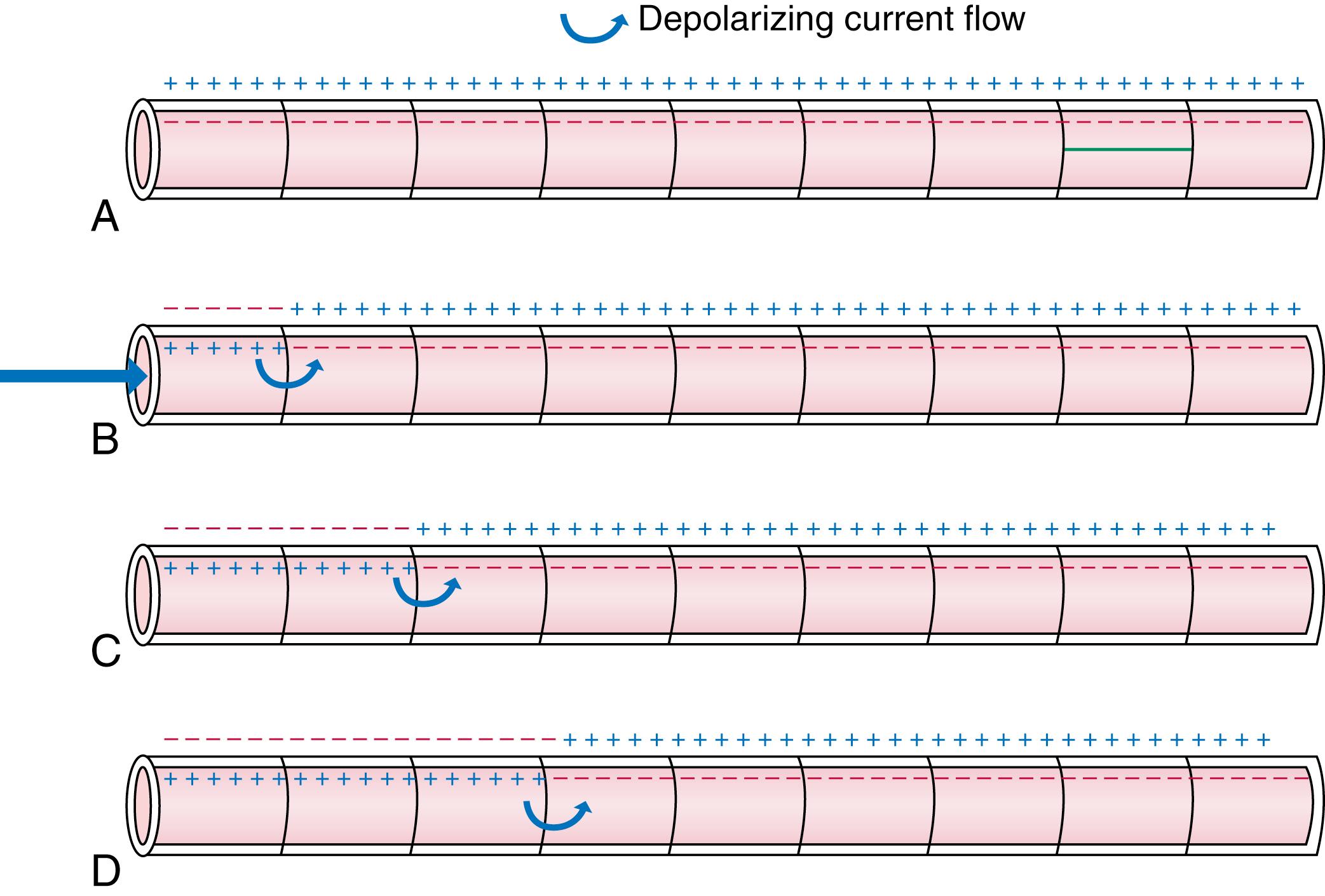
In addition to the active electrical properties that govern APs, cardiac electrical function is also determined by passive electrical properties. When a cell membrane depolarizes, the Na + that rushes in changes the local transmembrane potential quite strongly. Figure 62.7 illustrates how an electrical impulse travels along a cardiac fiber bundle composed of multiple cardiac cells (cardiomyocytes). Cardiomyocytes are electrically coupled to each other by low-resistance “gap junctions” (see below), making a bundle of cardiac fibers behave like a continuous cable. Panel A represents the resting bundle, with cardiomyocytes having a transmembrane potential of −80 mV due to more negative charges on the internal side of the membrane. Panel B illustrates what happens when an impulse arrives from an activated cell to the left, depolarizing the first cardiomyocyte in the bundle. The cell membrane, depolarized to threshold, undergoes phase 0 and changes rapidly from being relatively negative internally (−80 mV transmembrane potential) to becoming positive (+40 mV). A flow of electrical current (by definition, movement of positive ions) ensues as illustrated by the curved arrow in B, depolarizes adjacent membrane to threshold and causes cellular activation, as shown in C. The impulse then spreads further as shown in D, and eventually makes its way to the end of the bundle. One major determinant of impulse spread is how fast depolarizing current flow spreads from the activated region to the region that is not yet activated. When a depolarizing current is passed across an electrical resistor (like the lipid cell membrane), the voltage change produced depends on the resistance (by virtue of Ohm’s law, E = IR, where E = voltage change, I = current, R = resistance). Thus, membrane resistance is an important factor governing conduction speed in the heart. The resting fast-response cardiac cell membrane has a relatively low resistance because of the high K + conductance through I K1 ; rapid conduction therefore requires a very large current flow (ensured by large phase 0 Na + current). Slow-channel tissue has a much higher resistance because of lack of I K1 , allowing the relatively small I CaL to produce conduction, albeit much more slowly than in fast-response tissue. Membrane resistance is a very important “passive” membrane property, as opposed to active membrane functions mediated by ion channels.
The changes in membrane permeability that mediate the phases of the cardiac AP are largely produced by the time- and voltage-dependent function of cardiac ion channels, the building blocks of biologic electricity in the heart, brain, skeletal muscle, and other excitable tissues. Specialized transmembrane glycoproteins form ion channels: ion-selective pores in cell membranes that open and close (gating) in response to an appropriate biologic signal. The ion channels most centrally involved in controlling cardiac AP gate in response to changes in transmembrane voltage (“voltage-gated channels”). Other physiologically important channels respond to chemical ligands such as ACh, ATP, and Ca 2+ . Electrophysiologic studies have detailed the functional properties of Na + , Ca 2+ , and K + currents in cardiomyocytes, and molecular cloning has revealed a large number of pore-forming (α) and auxiliary (β, γ, and δ) subunits that form ion channels. Mutations in the genes encoding cardiac ion channel subunits are responsible for many forms of inherited cardiac arrhythmias (see Chapter 63 ). The expression and functional properties of myocardial ion channels also change in a number of acquired disease states, and these alterations can predispose to cardiac arrhythmias.
Voltage-gated Na + (Nav) channel pore-forming (α) subunits have four homologous domains (I to IV), each of which contains six-transmembrane–spanning regions (designated S1 to S6), and these four domains come together to form the Na + -permeable pore ( Fig. 62.8A ). Among the multiple Nav α subunits, Nav1.5 (which is encoded by the SCN5A gene) is the most strongly expressed in mammalian myocardium. The name of the voltage-gated sodium channel protein consists of the chemical symbol of the principal permeating ion (Na + ) and v, which indicates its principal physiologic regulator (voltage). The number following v indicates the gene subfamily (Nav1), and the number following the decimal point identifies the specific channel isoform (e.g., Nav1.1). An identical nomenclature applies to voltage-gated calcium and potassium channels. Mutations in SCN5A , which are associated with LQT3 syndrome, disrupt Nav channel inactivation and thereby give rise to a sustained inward Na + current during the plateau phase of the AP and to APD prolongation. Mutations in SCN5A are also linked to Brugada syndrome. Brugada syndrome mutations result in reduced I Na amplitude, which leads to slowing of the phase 0 AP upstroke, reduced AP amplitude, and altered phase 1 early repolarization.
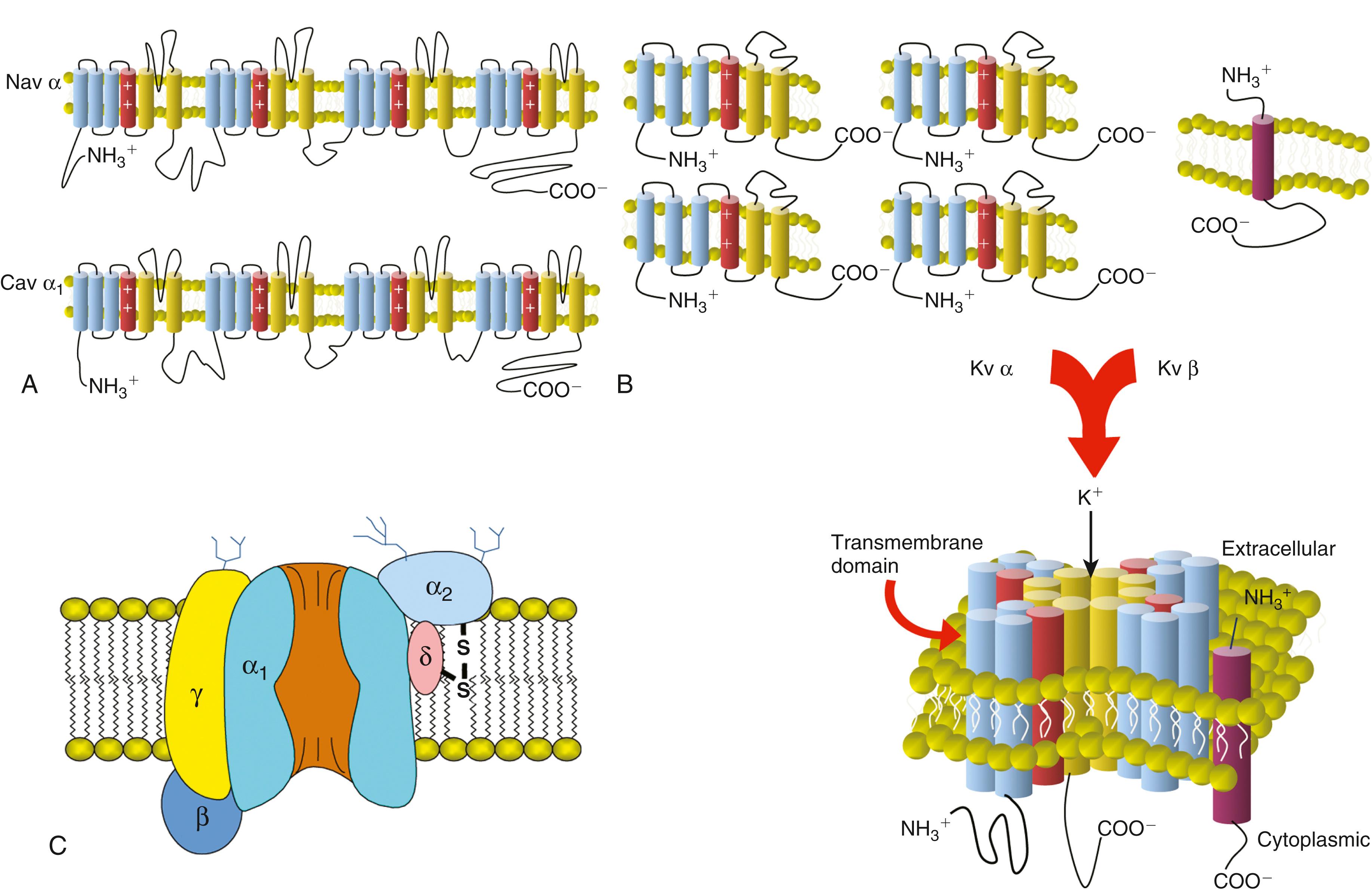
Nav1.5 pore-forming α subunits coassemble with one to two auxiliary Nav β subunits to form functional cell-surface Na + channels in cardiomyocytes. Nav β subunits appear to play an important role in anchoring ion channel proteins to the cell membrane. Subpopulations of Nav1.5 channels are present in different subcellular regions, such as the intercalated disc and T-tubular membranes. As with many other ion channels, Nav1.5 channels are part of macromolecular complexes including both channel and regulatory proteins. ,
As with Nav channels, cardiac voltage-gated Ca 2+ (Cav) channels are assemblies of a pore-forming α 1 subunits and auxiliary Cav β or Cav α 2 −δ subunits (see Fig. 62.8C ). Among the various α subunits, Cav1.2, also known as α 1C encoded by the CACNA1C gene, is the prominent Cav α 1 subunit expressed in mammalian myocardium. Cav1.2 channels exhibit many of the time- and voltage-dependent properties and pharmacologic sensitivities of cardiac L-type Ca 2+ currents (see Table 62.1 ). Cav1.3 channel subunits may also form L-type Ca 2+ channels, particularly in the SA node and atria. Accessory subunits modulate the functional properties of Cav channels.
Cav3.1/α 1 G alpha subunits form a Ca 2+ -selective channel with time- and voltage-dependent characteristics and pharmacologic sensitivities that resemble those of the low-voltage activated T-type Ca 2+ channel. Disruption of the gene encoding Cav3.1 subunits (CACNA1G) in mice slows the sinus node rate and AV conduction, and reduces the heart-rate response to adrenergic stimulation, consistent with a role in SA and AV node function.
Voltage-gated K + channels (Kv) are the most diverse family of voltage-dependent channels in the heart. Kv channels are composed of four separate pore-forming (α) subunits, each containing six-membrane–spanning domains (S 1 through S 6 ) (see Fig. 62.8B ). Kvα subunits expressed in the human heart include members of the Kv1, Kv4, hERG (Kv7), and KvLQT (Kv11) subfamilies. In addition, Kv channel α subunit proteins interact with Kv channel accessory subunits, including minK, KChIP2, and MiRP1 (see Table 62.1 ), to form functional cell surface channels with distinct time- and voltage-dependent properties. Co-assembly of the Kv4.3 α subunits and the accessory subunit KChIP2 gives rise to the cardiac transient outward Kv channel I to (see Phase 1: Early Rapid Repolarization). hERG α subunits, possibly together with MiRP1 accessory subunits, form functional cardiac I Kr channels. Mutations in the gene encoding hERG (KCNH2) have been shown to underlie congenital LQT2 syndrome. These LQT2 mutations are loss-of-function mutations that lead to reduced functional I Kr channel expression or to alterations in channel processing or trafficking (see Chapter 63 ).
KvLQT1 α subunits associate with minK (encoded by KCNE1 ) accessory subunits to form channels that carry slowly activating, noninactivating K + currents, identified with I Ks in human myocardium. Mutations in the gene encoding KvLQT1 α subunits, KCNQ1 , are linked to LQT1 syndrome. Mutations in the minK-encoding gene KCNE1 are associated with LQT5 syndrome. These mutations are all loss-of-function mutations that reduce expression of functional I Ks channels in the cell membrane.
Kv1.5 α subunits contribute to K + -selective channels with time- and voltage-dependent characteristics that underlie the rapidly activating and slowly inactivating I Kur in human atrial myocytes. I Kur is downregulated in the atria of patients with chronic AF.
Small-conductance, Ca 2+ -sensitive K + channels are tetrameric assemblies of SK α subunits (encoded by KCNN1-3 ) and underlie a Ca 2+ -activated K + current, I KCa, in human cardiomyocytes. Common variants in KCNN3 discovered in genome-wide analyses have been associated with AF.
Kir channels in cardiac myocytes, as in other cells, conduct inward current at membrane potentials negative to E K (see earlier, Physiology of Ion Channels) and smaller outward currents (due to inward rectification) at membrane potentials positive to E K . The current through Kir channels is a function of both the membrane potential and the extracellular K + concentration ([K + ] o ) and is the major determinant of the resting membrane potential in working myocardium and the specialized ventricular conducting system. When [K + ] o increases, the E K moves to more positive values and the cell depolarizes.
Inward rectification reduces the permeability of K + channels at depolarized voltages to prevent too much K + leaving the cell. Mechanisms include channel block by binding of magnesium and positively charged organic ions to the channel (for I K1 ) and extremely rapid inactivation (for I Kr ). In contrast to Kv α subunits, Kir α subunits have only two (not six) transmembrane domains. The α subunits Kir2.1 and Kir2.2 encoded by KCNJ2 and KCNJ3, respectively, are the main subunits underlying the I K1 inwardly rectifying current in cardiomyocytes.
The K + -selective sarcolemmal I K.ATP channel is formed by the association of pore-forming Kir6 α subunits and sulfonylurea receptor (SUR) subunits. In cardiomyocytes, Kir6.2 α subunits (encoded by KCNJ11 ) assemble with SUR1 and SUR2 subunits encoded by ABCC8 and ABCC9, respectively, to carry I K.ATP. Different anatomic regions of the heart may express I K.ATP comprised of differing channel and SUR subunits. I K.ATP channels play a pivotal role in myocardial ischemia and preconditioning, by reducing APD and myocardial metabolic demands when cellular ATP content goes down. Opening of cardiac sarcolemmal I K.ATP channels underlies ST-segment elevation during acute myocardial ischemia. I K.ATP is an important regulator of the function of multiple tissues. For example, in blood vessels, I K.ATP leads to vasodilation and in the pancreas, I K.ATP inhibits insulin secretion. Drugs like nicorandil and diazoxide open ATP-sensitive K + channels, whereas sulfonylurea compounds (e.g., glibenclamide) inhibit I K.ATP . In addition to the sarcolemmal I K.ATP channel an ATP-sensitive potassium conductance in mitochondria (mitoK[ATP]) is involved in cardioprotection and arrhythmias. The molecular composition of this channel is uncertain but likely involves another type of inwardly rectifying K + channel.
The ACh-activated K + channel I K.ACh is a heteromultimer of two inwardly rectifying potassium channel subunits, Kir3.1 and Kir3.4. , Stimulation of I K.ACh by vagally secreted ACh hyperpolarizes SA and AV node cells, decreases the slope of spontaneous depolarization in the SA node, slowing heart rate, and slows conduction in the AV node. Adenosine, through type 1 purinergic receptor–mediated G-protein activation, opens the I K.ACh channel (in this context carrying a current referred to as I K.Ado ) in atrial, SA node, and AV node cells. Adenosine and vagal-enhancement maneuvers are useful for the acute termination of arrhythmias involving the AV node as part of the reentry circuit, such as atrioventricular reentrant (AVRT) and atrioventricular nodal reentrant tachycardias (AVNRT) (see later, Mechanisms of Arrhythmogenesis).
The I f pacemaker current prominently contributes to diastolic depolarization in all tissues with spontaneous automaticity. I f activates slowly at negative potentials and deactivates rapidly with depolarization, carrying a mixed monovalent cation (Na + and K + ) current. I f is highly regulated; beta-adrenergic stimulation increases the probability of channel opening by shifting the channel’s activation curve to more positive potentials, accelerating diastolic depolarization. The HCN channels underlying I f are topologically similar to voltage-dependent K + channels and related to cyclic nucleotide–gated channels in photoreceptors in the retina. Of the four known HCN pore-forming α subunits, HCN4 is the most highly expressed in the mammalian myocardium. Mutations in the human HCN4 gene have been linked to familial sinus bradycardia and inappropriate sinus tachycardia. I f -blocking drugs have been approved for the treatment of angina and are under investigation for the treatment of various forms of heart failure and arrhythmias.
The NCX is an ion transporter that exchanges three Na + ions for one Ca 2+ and is very strongly expressed in the mammalian heart. With each cycle, NCX exchanges three positive charges (Na + ions) for every Ca 2+ ion (two positive charges) that it handles, producing a net current in the direction of Na + movement (its function is therefore “electrogenic”). NCX almost always functions to extrude Ca 2+ , resulting in a net inward current. The cardiac NCX is a transmembrane glycoprotein proposed to have nine-transmembrane repeats based on hydropathy analysis ( Fig. 62.9A , B ) . The intracellular loop contains domains that bind Ca 2+ (CBD 1 and 2) and the endogenous NCX inhibitory domain, XIP.
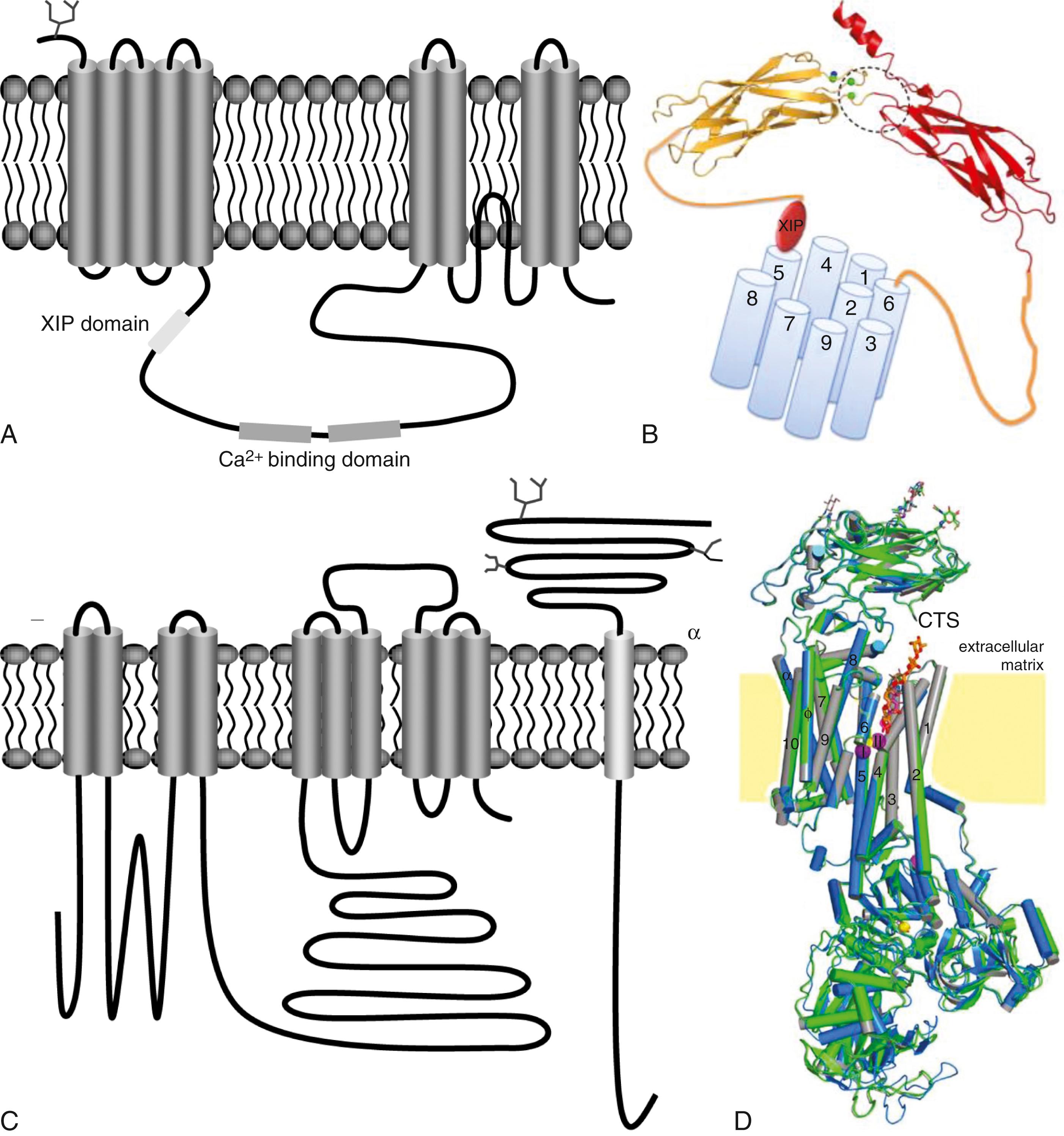
Ion exchange through NCX can occur in either direction. With each heartbeat, the phase-2 entry of Ca 2+ triggers a large cytosolic Ca 2+ release from SR stores through the release-channel RyR2. Intracellular [Ca 2+ ] then increases from the resting level of less than 100 nM to approximately 1 μM. Outward Ca 2+ flux through the NCX (in exchange for Na + , generating an inward current) along with Ca 2+ reuptake into the SR by the SR Ca 2+ -ATPase (SERCA) remove cytosolic Ca 2+ during diastole, restoring diastolic [Ca 2+ ] to its resting level. NCX current is time-independent and largely reflects changes in intracellular [Ca 2+ ] during the AP. Thus, NCX can contribute to determining the transmembrane voltage. At depolarized potentials, reverse-mode Na + /Ca 2+ exchange (Ca 2+ influx, net outward current) can occur; however, the role of reverse-mode exchange in initiating SR Ca 2+ release and contraction is uncertain.
NCX current is an important component of the inward current that underlies delayed afterdepolarizations (DADs). DADs are spontaneous membrane depolarizations after complete repolarization of the AP. They generally result from spontaneous diastolic SR Ca 2+ release events under pathologic conditions. The cytosolic Ca 2+ transient resulting from Ca 2+ release causes Ca 2+ to be extruded via NCX in exchange for extracellular Na + release, producing a depolarizing inward current. When the resulting membrane depolarization reaches threshold it initiates an ectopic AP; repeated DADs can cause or trigger tachyarrhythmias.
Also called the Na + pump, Na + ,K + -ATPase maintains the high intracellular K + and low intracellular Na + concentration of cardiomyocytes. The Na + pump belongs to the widely distributed class of P-type ATPases cation transporters. The P-type designation refers to the formation of a phosphorylated aspartyl intermediate during the catalytic cycle. The Na + ,K + -ATPase hydrolyzes a molecule of ATP to transport two K + into the cell and three Na + out and thus is electrogenic, generating a time-independent hyperpolarizing outward current. The Na + ,K + -ATPase is oligomeric, consisting of α and β subunits and a tissue-specific regulator protein called phospholemman (PLM). PLM belongs to a family of single-membrane–spanning proteins called FXYD proteins, which share a characteristic 35-amino acid sequence including a PFXYD (proline-phenylalanine-X-tyrosine-aspartic acid) sequence in their extracellular domain. PLM (FXYD1) is expressed in heart and skeletal muscle, which binds to and inhibits Na + ,K + -ATPase, acting as an important endogenous regulator.
Na + ,K + -ATPase isoforms are diverse and exhibit tissue-specific distributions. The structural diversity of the Na + ,K + -ATPase comes from variations in α and β genes, splice variants of the α subunits and promiscuity of subunit associations. The α subunit is catalytic and binds digitalis glycosides in the extracellular linker between the first and second membrane-spanning region ( Fig. 62.9C and D ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here