Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Although spinal cord tumor and spinal cord arteriovenous malformation (AVM) operations may appear to be unrelated, they are, in fact, closely linked. Both tumors and AVMs are space-occupying lesions, present challenging technical scenarios, and require an understanding of their natural history to pursue the wisest course of treatment. Addressing the mass lesion while preserving blood supply to the spinal cord is a goal in treating both tumors and AVMs. As a result of advanced magnetic resonance imaging (MRI), angiography, endovascular techniques, and neurophysiologic monitoring, more aggressive surgeries can safely be performed, with improved outcomes relative to the earliest reports.
The first reported attempt to resect an intramedullary spinal cord tumor was described in 1890. The patient died in the early postoperative period. As a result, these tumors were considered essentially nonsurgical lesions until the first described successful resection of an intramedullary ependymoma in 1907 by Anton von Eiselsberg. After undergoing a gross total resection, the patient regained and maintained ambulation with long-term follow-up.
As the new wave of intramedullary spinal cord tumor surgery progressed, Elsberg and Beer advocated attempts at gross total resection by identifying safe tissue planes with blunt dissection. They reported an aborted first attempt at resection of an intramedullary tumor in which hemodynamic instability was encountered after myelotomy was performed. Upon returning to the operating room 1 week later, Elsberg found that the tumor had extruded through the myelotomy defect with minimal spinal cord manipulation, and the patient regained function. Subsequently, Elsberg advocated such a two-stage approach for all intramedullary tumors in his seminal text, Diagnosis and Treatment of Surgical Diseases of the Spinal Cord and Its Membranes, in 1916. Although there are still many surgeons who practice this “two-stage” approach, technological advances have allowed many more intramedullary lesions to be resected with good outcomes in one stage. Further series demonstrated reliably that ependymomas are more likely to be safely completely resected, whereas astrocytomas frequently lack a necessary clear plane between neoplastic and normal tissue ( Table 175.1 ).
| Patient | Age (Year) | Location | Histology | Removal | Result | Follow-up |
|---|---|---|---|---|---|---|
| 1 | 26 | C | Ependymoma | T | I | 3 months |
| 2 | 24 | Conus | Ependymoma | T | I | 1 year |
| 3 | 49 | C | Ependymoma | T | I | 5 years |
| 4 | 28 | C-D | Ependymoma | T | S | 4 years |
| 5 | 38 | C | Ependymoma | T | S | 4 years |
| 6 | 45 | Conus | Ependymoma | T | S | 2 years |
| 7 | 35 | Conus | Ependymoma | T | S | 2 years |
| 8 | 20 | Conus | Ependymoma | T | S | 2 years |
| 9 | 35 | C | Ependymoma | T | I | 1 year |
| 10 | 12 | C | Astrocytoma | P | S | 6 months |
| 11 | 60 | C-D | Astrocytoma | 90% | S | 6 years |
| 12 | 13 | D-L | Astrocytoma | T | S | 4 years |
| 13 | 3 | C-D | Astrocytoma | 95% | I | 4 years |
| 14 | 48 | C | Astrocytoma | T | I | 4 years |
| 15 | 38 | C | Astrocytoma | T | I | 5 years |
| 16 | 7 | C | Astrocytoma | T | I | 1 year |
| 17 | 5 | C-D-L-S | Astrocytoma | 50% | S | 1 year |
| 18 | 14 | C | Malignant glioma | P | W | 1 (died) |
| 19 | 20 | C | Malignant glioma | P | W | 6 months (died) |
| 20 | 13 | D | Teratoma | T | I | 4 years |
| 21 | 12 | Conus | Dermoid | T | I | 11 years |
| 22 | 4 months | Conus | Epidermoid | P | I | 11 years |
| 23 | 28 | D-L | Mixed | P | W | 2 years |
| 24 | 52 | D | Metastatic | P | S | 1 month (died) |
| 25 | 57 | C | Hemangioblastoma | T | I | 2 months |
Technical advances account for a large part of the reason that intramedullary tumors are now seen as surgically treatable diseases. The development of the binocular operating microscope in the 1920s, and its first neurosurgical use by Theodore Kurze, revolutionized all aspects of neurosurgery. It has become absolutely mandatory in the resection of intramedullary lesions.
Bipolar cautery was first developed in the 1930s and expanded in use with Leonard Malis. It allowed for the precise coagulation of tumor vessels while sparing vessels supplying the cord. It also directed electrical current between two forceps tips, protecting surrounding neural tissue from thermal injury. It is indispensable in identifying the natural tumor/cord cleavage plane, if one exists.
Myelography was the “gold standard” in diagnosis of spinal cord tumors until the advent of MRI. MRI was developed in the 1970s by teams of scientists and was being applied to the spinal cord by the 1980s. Previously, indirect evidence of spinal cord tumor included cord expansion or myelographic block. With MRI, the spinal cord and tumor tissue could be directly visualized and described by their signal characteristics. As a result, preoperative diagnosis and planning can often be accomplished and help to direct the care of the patient. MRI is also useful in distinguishing nonneoplastic and nonsurgical lesions from surgical lesions, sparing unnecessary risk for many patients. Preoperative diffusion tensor imaging (DTI) tractography may be able to help determine which tumors will have a clear plane of demarcation with nonneoplastic tissue and which may then safely be resected. , Intraoperative imaging with ultrasound can help to localize the optimal site for myelotomy, and intraoperative MRI may eventually be helpful to assess the extent of resection.
Intraoperative neurophysiologic monitoring plays a crucial role in the safe resection of intramedullary lesions. Somatosensory evoked potentials (SSEPs), used intraoperatively since the 1970s, record the electrical conductivity along the dorsal columns. Thus they can be used to help identify the midline myelotomy site if the gross anatomy and visual landmarks are found to be distorted. Once the myelotomy is made, it is not unusual to lose SSEP recording, which typically correlates with a loss of vibration and proprioception in the appropriate limbs. With time and rehabilitation, patients learn to compensate for this lost function and can resume normal activities. Use of intraoperative SSEP prior to making the myelotomy may allow for precise identification of the midline between the two dorsal columns and may decrease the morbidity from injury to this tract.
Motor evoked potentials (MEPs) are also recorded throughout surgery. Peripheral muscle electrodes can record activity resulting from transcranial stimulation in an “all-or-none” fashion and lead to visible motion of the patient. Thus these can be recorded only intermittently and may be late in heralding the loss of function caused by a specific maneuver during surgery. Loss of peripheral muscle MEP not due to technical monitoring issues typically correlates with a temporary or permanent postoperative loss of motor function.
Transcranial MEP can also be recorded by an epidural electrode located distal to the resection level. This measures the D wave in a quantitative manner, allowing for graded rather than all-or-none interpretation of the electrical signals. It can be recorded with no detectable muscle movement and thus can be monitored continuously during resection. A drop in amplitude greater than 50% is considered significant and warrants a pause in the surgery, at least until signals recover. A surgeon may aggressively seek out a dissection plane as long as the D wave remains stable. Typically, a loss of peripheral muscle MEP with preserved epidural D wave indicates a transient loss of function that may be severe but is likely to recover. ,
In 1962, Turnbull stated: “A surgeon exploring a spinal cord for a suspected intramedullary tumor must be prepared to face a formidable problem and also have the courage of conviction to make every attempt to remove the tumor. Anything less than this, with a cursory inspection of the spinal cord or aspiration thereof, can only create problems of a more complex nature for the subsequent surgical effort to remove such tumors.” These words remain true and are the guiding principle in current intramedullary spinal cord tumor surgery.
Surgery remains the primary treatment for intramedullary tumors. Radiotherapy has little to offer as a primary treatment, even for malignant tumors. Radiation treatment has its most significant role as adjuvant treatment of World Health Organization (WHO) grades III and IV tumors. , Regarding the timing of surgery, there is little to be gained by allowing further tumor growth prior to offering surgery. The surgical results are generally predicated on the preoperative condition of the patient, no matter how large the tumor. If the patient preoperatively has minimal neurologic findings, then the postoperative course should be gratifying, especially if the tumor can be resected grossly. Those patients who arrive for surgery with significant preoperative deficits may regain little of their lost function even after a technically successful operation.
The prone position is generally used for all intramedullary cord tumors, although early in surgical experience, the sitting position was used for some cervical cord tumors. The prone position is currently preferred because it decreases the potential for vasomotor collapse, which can be significant when the autonomic pathways are compromised by the tumor or surgery; it also allows the assistant to take a more active role in the operation, providing traction, irrigation, or assistance with the dissection. General anesthesia with endotracheal intubation is always used, and the endotracheal tube may be left in place for 24 to 48 hours after surgery in patients with more extensive cervical intramedullary tumors. Electrodes are placed to monitor MEPs and SSEPs.
The full extent of the tumor must be known before surgery. This usually is well demonstrated on the preoperative MRI. Intraoperatively, once the dura is exposed, ultrasonography may be used to identify the extent of the solid tumor and associated cystic components. In the case of a suspected hemangioblastoma, the vasculature may be demonstrated by arteriography to aid in the surgical removal, but often the MRI is adequate. A wide laminectomy is then performed over the entire extent of the solid portion of the tumor, extending to a level above and below it. The dura must be opened carefully because the pia-arachnoid of the enlarged spinal cord often adheres to the underside of the dura. It is important to minimize any injury to the cord vasculature, because hemorrhage during this phase of the operation will obscure all landmarks and significantly compromise the surgical effort. In the event of previous surgery, particularly if the dura has been left open, this early dissection is tedious, but care must be taken to reestablish all anatomic landmarks. In those cases, either with or without previous surgery, the initial appearance of the cords generally is similar. The spinal cord appears widened, often without evidence of tumor on the surface. The widening of the spinal cord and the presence of dilated veins at the caudal end of the tumor site are indications of the underlying pathologic process. Ultrasound may help to confirm this. When observing a widened spinal cord, the surgeon must not be misled in those rare instances of anterior extramedullary lesions in which the cord is splayed out over the lesion.
The dorsal surface of the cord at the area of greatest enlargement is then inspected for the site of the myelotomy. In general, myelotomies are performed in the midline with a preference for the thinnest and most avascular areas. When the spinal cord is rotated and the midline is difficult to identify, the bilateral dorsal root entry zones can be identified and thereby help to define the midline to perform the myelotomy. Intraoperative dorsal column monitoring can be useful in determining the location for the midline myelotomy. However, at times, it is more expeditious to use a paramedian approach ( Fig. 175.1 ).
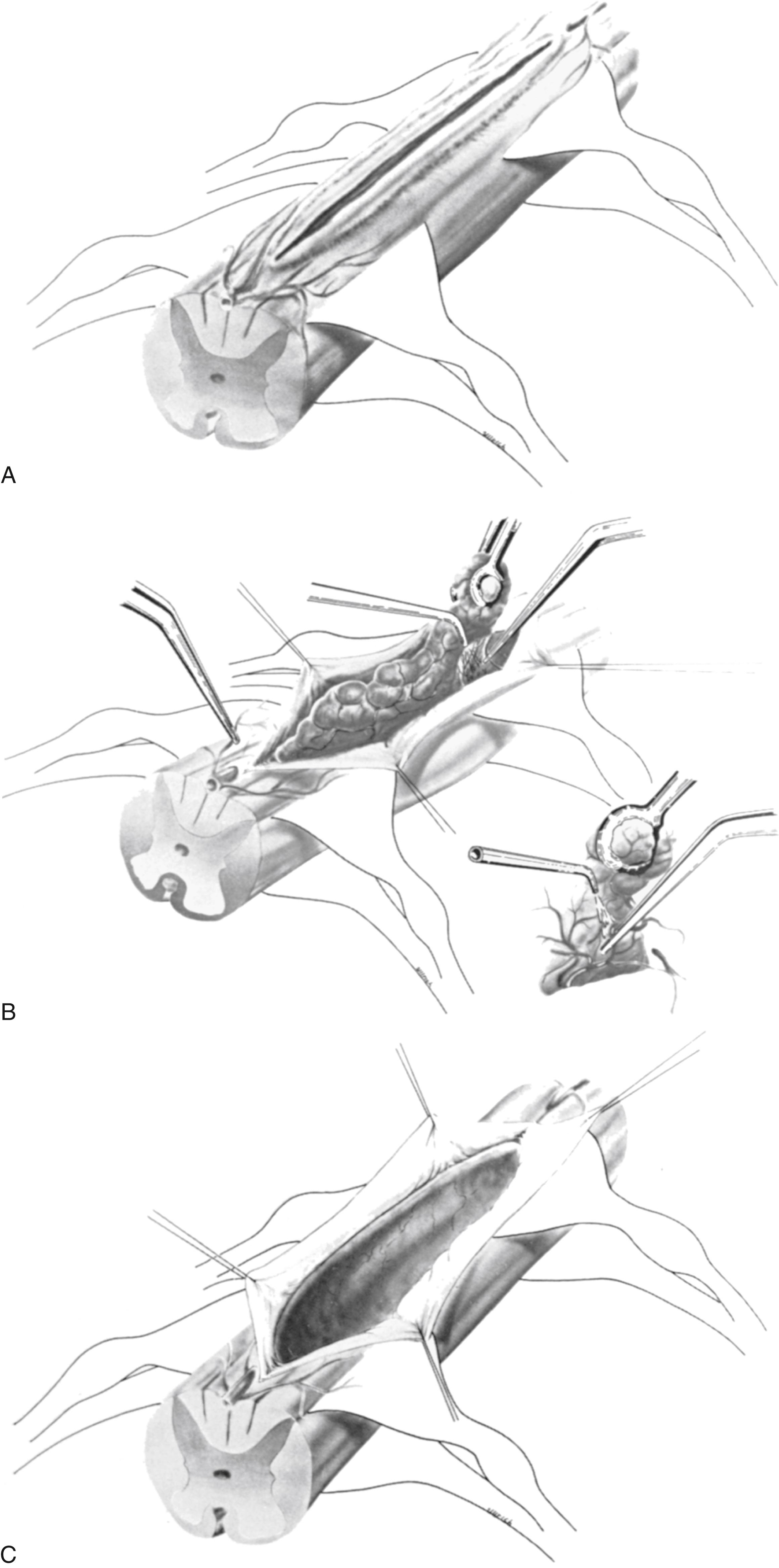
Some of the vasculature will be sacrificed during this type of myelotomy. An initial incision of approximately 1 to 2 cm is made over the greatest enlargement of the spinal cord to evaluate the plane between the tumor and the spinal cord tissue. In some instances, the presence of a cyst associated with the tumor may be easily noted; if so, additional room may be gained by aspirating some of the cystic contents through a small-bore needle. However, to facilitate dissection, these cysts should not be completely evacuated. Once it is determined that the neoplasm is cystic or has well-defined planes, the myelotomy is lengthened over the extent of the tumor. With teratomas, a stalk between the cord and the dura should be removed as an integral part of the lesion. With ependymomas extending from the conus, the extra-axial portion in the cauda equina may be removed first, providing adequate decompression and visualization of the residual tumor involving the intramedullary portion of the conus. The draining veins of intramedullary hemangioblastomas must be left until the latter part of the surgical resection.
Following the myelotomy, 6-0 or 7-0 traction sutures are placed through the pial margins on either side and sutured to the edges of the opened dura to expose the interior of the spinal cord (see Fig. 175.1 ). In general, the tumor is visible a few millimeters under the dorsal surface of the spinal cord. There is often a soft gliotic interface between the tumor and the spinal cord proper. With the use of an operating microscope, microsurgical techniques, bipolar cautery, small suction tubes, and various dissectors, a plane is developed around the margin of the tumor, taking care to retract primarily on the tumor and not on the spinal cord. Gentle use of plated bayoneted forceps can help to tease out natural planes between normal and neoplastic tissue. The surgeon works to one or the other end of the tumor. At the pole where the tumor is the narrowest, it may be possible to grasp the end and gently extract it from the interior of the cord. All the fine vascular adhesions to the spinal cord, especially on the ventral aspect of the tumor, should be cauterized with bipolar cautery and sharply divided. No blunt dissection should be carried out in areas where vascular channels connect the tumor to the spinal cord. Most tumors are relatively avascular and present little of hemorrhage or the loss of control of large blood vessels. It is important to keep the operative field meticulously dry so that the plane between the tumor and the spinal cord may be readily identified (see Fig. 175.1 ).
If the tumor is too large to remove en bloc or necessitates too much retraction on the spinal cord, its interior may be decompressed. The ultrasonic aspirator may be used to debulk the tumor, facilitating dissection around its capsule and its removal. This has a minor disadvantage of spilling the contents of the tumor into the dissection plane or obscuring the dissection plane by bleeding. However, in most instances, because the tumors are relatively avascular, bleeding is not a major problem, even with the use of the ultrasonic aspirator. Gradually, the entire tumor may be removed. For those tumors (usually astrocytomas) that are infiltrating, a debulking procedure may be valuable, especially in children. Carbon dioxide and argon lasers have also been used for this purpose. More recently, the development of sapphire-tipped Nd:YAG (neodymium:yttrium-aluminum-garnet) and argon lasers has allowed very focused transmission of energy located at the tip itself. This can be useful in creating the initial myelotomy and in dissecting along the tumor-normal interface, with minimal manipulation of the spinal cord.
Cystic collections at the margins of the tumor, as in the case of ependymomas, facilitate the tumor resection. No attempt should be made to remove the wall of the cyst, which is thin and nonneoplastic. If there is any doubt about the totality of removal, small biopsies may be obtained from the margin of the resection and evaluated by frozen sectioning during surgery. In most instances, the margins are well defined and there is no question about the removal of the tumor (see Figs. 93.4 and 93.6). Malignant tumors such as the glioblastoma respond poorly to surgery and do not appear to respond to radiation therapy. In such cases, heroic lifesaving efforts at cordotomy have been attempted, with dismal results. ,
In children, the syndrome of halo spinal cord widening is associated with extensive astrocytomas or localized astrocytomas and extensive cysts. In these cases, one must localize the tumor through either clinical or radiographic evaluation and remove it while draining only the cyst. A subtotal radical removal with the ultrasonic aspirator or laser appears to pay dividends in these children, with long-term remission of the disease process. Although these astrocytomas are histologically identical to those in adults, they may have different growth behavior related more to the child’s development than to the histologic appearance.
Special attention is given to the removal of hemangioblastomas. These are highly vascular tumors, and, if they are decompressed or cut into, the bleeding will obscure the anatomic planes. Therefore, even in large hemangioblastomas, the surgeon works around the margin of the tumor, interrupting the feeding arteries and finally the primary draining vein as the tumor is rolled out on the last venous pedicle. Often these tumors are associated with a cyst, facilitating their removal. They are identified by their characteristic orange-red appearance and in all instances extrude from the pial surface. Their presence is also identified by large, dilated, arterialized veins that often surround them. This may mimic an AVM on myelograms and spinal angiograms. In some instances, hemangioblastomas may be multiple, and additional tumors may be removed if they are accessible. In rare instances, they are associated with cranial tumors or von Hippel-Lindau syndrome.
In cases in which the tumor has previously been treated with radiation, intense intramedullary gliosis may be noted at biopsy, distinctly separate from the margin of the tumor, and usually at the caudal or rostral interface. Previous surgery with aspiration of a cyst but without definitive removal of the intramedullary tumor may provide transient benefits and eventual reaccumulation of the cyst fluid.
In intramedullary teratomas, the border of the tumor, although well defined, may be densely adherent to the surrounding spinal cord. Every attempt should be made to remove this capsule, which is a potential source of regrowth. There may be extensive involvement of central areas of the spinal cord, and the tumors may extend from the posterior to the anterior surface. Teratomatous tumors also may have a dumbbell configuration within the substance of the spinal cord, and the surgeon must be wary not to miss satellite portions of the primary tumor.
When the margins of the cord are allowed to fall back into position, the remarkable decompressive effect of tumor removal is quite apparent. The remaining cord may be thinned to the point of being transparent. If there has been minimal retraction on the cord, these fiber tracts remain preserved in a satisfactory way and will show progressive functional recovery. Gentleness of dissection may be gauged by the vascular pattern on the dorsal surface of the cord at the completion of tumor resection. Distended veins that were present before removal, usually at the caudal end of the tumor on the surface of the spinal cord, will not be less prominent. MEP and SSEP monitoring should be stable. No effort is made to sew the pial surfaces of the spinal cord together. The dura is closed in a watertight fashion; it is rarely necessary to use a dural substitute other than in those cases in which the dura was left open after previous surgery. If total removal of an intramedullary tumor is not feasible, the dura should be reconstructed, preferably with a fascial graft, to decompress the spinal cord and so that subsequent surgical endeavors may be facilitated.
An important factor that determines the ease of the operation is the presence and nature of previous operations. In patients who have had previous surgery and in whom the dura mater was left open, the initial exposure of the tumor and the dissection of adjacent tissues from the spinal cord may prolong the operation and make exposure more difficult. In those cases in which radiation was administered previously, gliotic areas in the dorsocentral portion of the spinal cord will have been verified by biopsy. There will have been no histologic changes in the tumor that were attributed to radiation. Similarly, none of the tumors that were previously irradiated showed malignant changes. Problems with wound healing also were encountered frequently in those cases in which radiotherapy was used previously.
A number of aspects concerning the surgical removal and management of intramedullary spinal cord tumors must be emphasized. The surgeon should assume that the majority of all intramedullary cord tumors are benign and resectable. Intraoperative judgment should be based on the gross appearance rather than the histologic tumor characteristics, because some low-grade astrocytomas are well-circumscribed and resectable. An inadequate myelotomy may fail to reveal a clear plane, and frozen section specimens made from a small piece of tumor taken through a limited myelotomy may lead to an erroneous tissue diagnosis and thus be misleading. This problem often occurs with tanycytic ependymomas, which may be mistaken for astrocytomas on frozen sections. Therefore, if a clear plane is identified between tumor and normal tissue, the surgeon should continue with the resection as long as electrophysiologic monitoring is stable. On the other hand, if the frozen histologic diagnosis is ependymoma, then every attempt must be made to identify a plane, even if one is not initially obvious, and to remove the tumor in total. This is particularly true for large tumors that initially seem infiltrative or that severely compress the surrounding spinal cord such that it is almost unrecognizable. It is sometimes amazing that this thin ribbon of cord tissue can function reasonably well and recover some of its lost function. Therefore even very large and extensive ependymomas should not deter the surgeon from complete removal. Numerous studies have demonstrated that identification of dissection planes is a major factor in determining the completeness of resection and the eventual long-term prognosis for patients with such tumors.
Although intramedullary tumors occur most commonly in adults, a significant incidence has been reported in children. , The symptomatology in both age groups is similar. Slowly growing tumors may displace much of the spinal cord substance before becoming symptomatic, but ultimately an end point is reached when compensatory ability fails and marked neurologic deterioration develops rapidly. Persistent pain involving the dorsal root dermatomes in the area of tumor involvement is often the signature of an intramedullary neoplasm. Dysfunction of the posterior column may occur in a progressive fashion, with sensory dysesthesias in the arms, torso, and legs, depending on the site of the intraspinal neoplasm. Sacral sparing may be present and is not an invariable finding with intramedullary neoplasms. Lower motor neuron symptoms and signs usually occur at the level of the tumor. Well-defined central cord syndromes, such as those seen in syringomyelia, with disassociated sensory loss and the classic signs of anterior horn cell involvement may be lacking. Children may present with scoliosis. The symptomatology generally is progressive, with few remissions or exacerbations. Symptoms are usually bilateral, but in rare instances neurologic abnormalities are confined to one extremity. The duration of symptoms generally is measured in years, although some neoplasms may have histories of 6 months or less.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here