Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
In the past, it was believed that most malabsorptive diseases manifested with diarrhea, steatorrhea, or both. It is now recognized that many malabsorptive disorders, such as celiac disease, might have subtle clinical presentations or mainly extraintestinal manifestations (e.g., anemia, bone loss, menstrual disturbance) that lead to a delayed and/or erroneous diagnosis. Awareness is also increasing that subtle malabsorption of single nutrients such as calcium or vitamin B 12 can, if unrecognized, lead to complications that may be difficult to reverse or that are even irreversible. The clinical challenge today is to recognize and treat malabsorption despite its subtle manifestations, a challenge made even more difficult by the restricted availability of tests for malabsorption, such as the 72-hour fecal fat determination.
Classically, maldigestion is defined as defective intraluminal hydrolysis of nutrients, and malabsorption is defined as defective mucosal absorption. Although this distinction may be useful on pathophysiologic grounds, the clinical presentation and complications of maldigestion and malabsorption are similar. Moreover, physiologic processes other than digestion and absorption (e.g., solubilization, intestinal motility, hormone secretion) contribute to the normal absorption of nutrients, vitamins, and minerals, so the classic definitions of maldigestion and malabsorption do not cover the actual pathophysiologic spectrum of the malabsorption syndrome. In this chapter, the terms digestion and absorption or maldigestion and malabsorption are used separately only in the discussion of pathophysiology. When the distinction between these terms is not of clinical relevance, only the terms absorption and malabsorption are used.
Malabsorption can be caused by many diseases of the small intestine as well as by diseases of the pancreas, liver, biliary tract, and stomach ( Box 104.1 ). In some of these diseases, malabsorption may be the presenting feature; in others, malabsorption may be only a minor clinical problem or detected only as a laboratory abnormality.
Atrophic gastritis
Autoimmune gastritis (pernicious anemia)
Gastric resection or bypass surgery
Congenital pancreatic enzyme deficiencies
Co-lipase deficiency
Lipase deficiency
Trypsinogen deficiency
Pancreatic insufficiency
Chronic pancreatitis
Cystic fibrosis
Johanson-Blizzard syndrome
Pearson marrow-pancreas syndrome
Shwachman syndrome
Pancreatic tumors
Inborn errors of bile acid biosynthesis and transport
Cirrhosis and other liver diseases
Portal hypertension
Biliary tumors
Primary and secondary sclerosing cholangitis
Amyloidosis
Autoimmune enteropathy
Celiac disease
Collagenous sprue
Congenital intestinal defects (see Table 104.10 )
Crohn disease
Enteroendocrine cell deficiency
Autoimmune Polyendocrinopathy, Candidiasis, EctodermalDystrophy (APECED)
Enteric anendocrinosis
Enterokinase deficiency
Eosinophilic gastroenteritis
Fistulas
Food allergy
Graft-versus-host disease
Hypolactasia
Ileal bile acid malabsorption
Intestinal infections
AIDS (HIV infection): cryptosporidiosis, Mycobacterium avium complex infection, viral infections
Giardiasis
Helminthic infections
Tuberculosis
Whipple disease
Immunoproliferative small intestinal disease
Intestinal ischemia
Intestinal lymphoma
Intestinal resections or bypass
Mastocytosis
Nongranulomatous chronic idiopathic enterocolitis
Postinfection malabsorption
Primary immunodeficiency diseases
Radiation enteritis
Refractory sprue
Sarcoidosis
SIBO
Tropical sprue
Primary intestinal lymphangiectasia
Secondary intestinal lymphangiectasia
Lymphoma
Solid tumors
Thoracic duct trauma, damage, or obstruction
Carcinoid syndrome
Gastrinoma (ZES)
Glucagonoma
Somatostatinoma
Constrictive pericarditis
Heart failure
Addison disease
Diabetes mellitus
Hyperthyroidism
Cronkhite-Canada syndrome
Mixed connective tissue disease
Neurofibromatosis type 1
Protein-calorie malnutrition
Scleroderma
SLE
This chapter provides an overview of basic pathophysiologic mechanisms that lead to symptoms or complications of maldigestion or malabsorption, reviews the clinical manifestations and complications of malabsorption, describes tests that can be used clinically to evaluate digestive and absorptive function, presents a rational diagnostic approach to the individual patient, and discusses malabsorptive diseases and general measures in the treatment of malabsorption syndrome not covered in other chapters of this book.
From a pathophysiologic point of view, mechanisms that cause malabsorption can be divided into pre-mucosal (luminal), mucosal, and postmucosal (vascular and lymphatic) factors. For clinical purposes, this approach is of limited value because the various clinical pictures caused by malabsorption syndromes are determined mainly by the nature of the malabsorbed substrates. We, therefore, discuss the mechanisms causing malabsorption based on the malabsorbed substrate. A separate section is devoted to the role of mechanisms that compensate for the consequences of malabsorption.
Normal uptake of nutrients, vitamins, and minerals by the GI tract (see Chapters 102 and 103 ) requires several steps, each of which can be compromised in disease.
Solubilization is a prerequisite for absorption of such nutrients as fat or calcium. Fat and fat-soluble vitamins are solubilized by the formation of micelles, and calcium is solubilized through acidification in the GI lumen. Alternatively, increased solubilization of the components of intestinal chyme can contribute to the manifestations of GI diseases, such as increased absorption of oxalate, which can result in the development of kidney stones in patients with short bowel syndrome (see Chapter 106 ).
Digestion of macromolecular compounds such as polysaccharides, triglycerides, and proteins to their molecular components—monosaccharides, fatty acids, and amino acids (AAs), respectively—is achieved by soluble or membrane-bound digestive enzymes. Absorption of undigested or partially digested macromolecular compounds occurs to a very minor degree in health and may be increased slightly in various intestinal diseases. Although such absorption does not play a nutritive role, it may be important for the normal function of the immune system and the pathogenesis of diseases such as food allergy (see Chapter 10 ).
Liberation of substrate (e.g., vitamin B 12 ) from binding sites in food or, conversely, binding to factors such as intrinsic factor (IF) allows absorption to take place.
Chemical changes to nutrients may be required for absorption, such as reducing the charge of iron from Fe +3 to Fe +2 .
Mucosal absorption can occur by active or passive carrier-mediated transport or simple or facilitated diffusion (see Chapter 101 ). Postmucosal transport of absorbed substrates occurs in blood vessels and lymphatic vessels.
Intestinal sensory and motor function permits detection of the presence of nutrients, facilitates adequate mixing of nutrients with intestinal secretions and delivery to absorptive sites, and provides adequate time for nutrient absorption (see Chapter 99 ).
Neural and hormonal functions are required to stimulate and coordinate digestive secretions, mucosal absorption, and intestinal motility (see Chapters 4 and 99 ).
An overview of pathophysiologic mechanisms of maldigestion and malabsorption is provided in Table 104.1 . This table also shows the ingested substrates primarily affected by individual pathophysiologic mechanisms and lists examples of etiologic disorders for these mechanisms.
| Pathophysiologic Mechanism | Malabsorbed Substrate(s) | Representative Causes |
|---|---|---|
| Maldigestion | ||
| Conjugated bile acid deficiency | Fat Fat-soluble vitamins Calcium Magnesium |
Hepatic parenchymal disease Biliary obstruction SIBO with bile acid deconjugation Ileal bile acid malabsorption CCK deficiency |
| Pancreatic insufficiency | Fat Protein Carbohydrate Fat-soluble vitamins Vitamin B 12 (cobalamin) |
Congenital defects Chronic pancreatitis Pancreatic tumors Inactivation of pancreatic enzymes (e.g., ZES) |
| Reduced mucosal digestion | Carbohydrate | Congenital defects (see Table 104.10 ) Acquired lactase deficiency |
| Protein | Generalized mucosal disease (e.g., celiac disease, Crohn disease) | |
| Intraluminal consumption of nutrients | Vitamin B 12 (cobalamin) | SIBO Helminthic infections (e.g., Diphyllobothrium latum infection) |
| Malabsorption | ||
| Reduced mucosal absorption | Fat Protein Carbohydrate Vitamins Minerals |
Congenital transport defects (see Table 104.10 ) Generalized mucosal diseases (e.g., celiac disease, Crohn disease) Previous intestinal resection or bypass Infections Intestinal lymphoma |
| Decreased transport from the intestine | Fat Protein |
Intestinal lymphangiectasia
Venous stasis (e.g., from heart failure) |
| Other Mechanisms | ||
| Decreased gastric acid and/or intrinsic factor secretion | Vitamin B 12 | Pernicious anemia Atrophic gastritis Previous gastric resection |
| Decreased gastric mixing and/or rapid gastric emptying | Fat Calcium Protein |
Previous gastric resection Autonomic neuropathy |
| Rapid intestinal transit | Fat | Autonomic neuropathy Hyperthyroidism |
For sufficient digestion and absorption of lipids, dietary fat must adequately mix with digestive secretions. Gastric resections or GI motility disorders that result in rapid gastric emptying or rapid intestinal transit, such as autonomic neuropathy due to diabetes mellitus or amyloidosis, can cause fat malabsorption consequent to impaired GI mixing of dietary fat.
Fat malabsorption resulting from decreased formation of micelles occurs if the luminal concentrations of conjugated bile acids (BAs) are lower than the critical concentration required for forming micelles. Table 104.2 details the pathophysiologic mechanisms and representative diseases that cause luminal BA deficiency.
| Pathophysiologic Mechanism | Causes |
|---|---|
| Decreased synthesis and/or secretion of conjugated bile acids | Parenchymal liver diseases (e.g., cirrhosis, PBC) |
| Biliary obstruction (e.g., tumors) | |
| Biliary fistulas | |
| Inborn errors of bile acid synthesis | |
| CCK deficiency | |
| Intestinal loss of conjugated bile acids | Ileal resection |
| Severe ileal mucosal disease | |
| Congenital defects of the ileal sodium-bile acid co-transporter | |
| Luminal deconjugation of bile acids | SIBO |
| Binding of bile salts or lack of solubilization of bile salts as a result of low luminal pH | Cholestyramine (binding) |
| ZES (low pH) | |
| Exocrine pancreatic insufficiency (low pH) |
If exocrine pancreatic function is severely reduced, impairment of pancreatic lipase and co-lipase secretion results in decreased luminal hydrolysis of dietary fat. Chronic pancreatitis, cystic fibrosis, pancreatic duct obstruction by pancreatic and ampullary tumors, and pancreatic resection are the most common causes of pancreatic insufficiency. Even when pancreatic enzyme concentrations are normal, reduced pancreatic lipase activity resulting from low luminal pH, excessive calcium ingestion, or ingestion of the specific lipase inhibitor orlistat can cause pancreatic steatorrhea. Selective congenital lipase or co-lipase deficiency is a rare cause of pancreatic fat malabsorption.
Generalized mucosal diseases such as celiac disease are often associated with fat malabsorption. Defective uptake of free fatty acids and monoglycerides results from reduced mucosal surface area secondary to villus shortening, reduced enterocyte function, and mucosal inflammation. Intestinal fat absorption is also impaired in diseases that result in disturbance of intracellular formation of chylomicrons and accumulation of lipids within the enterocytes, including abetalipoproteinemia, hypobetalipoproteinemia, and chylomicron retention disease.
Impairment of lymphatic transport of chylomicrons is a cause for postmucosal malabsorption of dietary fat. Decreased lymphatic transport can result from congenital diseases such as primary intestinal lymphangiectasia or from obstruction of lymphatic vessels as a result of metastatic solid tumors, lymphoma, Whipple disease, retroperitoneal fibrosis, or trauma (see Chapter 31 ). Usually, lymphatic vessels in the mucosa become dilated (lymphangiectasia), and chylomicrons are lost post-prandially into the intestinal lumen and also in the fasting state ; steatorrhea in these situations usually is only mild to moderate.
Defective digestion or absorption of dietary proteins has to be differentiated from excessive loss of serum proteins into the GI tract, which is termed protein-losing enteropathy (see Chapter 31 ).
Protein digestion may be impaired in patients who have undergone partial or total gastric resection, presumably as a result of poor mixing with digestive secretions, although gastric pepsin deficiency could be contributory. Impaired activation of pepsin due to acid inhibition by PPIs can result in an increased risk of food allergy with manifestations such as childhood asthma, owing to decreased gastric digestion of proteins (see Chapter 10 ). Defective proteolysis also occurs with exocrine pancreatic insufficiency. In congenital diseases, pancreatic proteolysis can be impaired by inborn errors in the synthesis of proteolytic enzymes (trypsinogen deficiency) or by defective activation of pancreatic proenzymes resulting from congenital deficiency of intestinal enterokinase (see Congenital Defects).
Generalized mucosal diseases, such as celiac disease, result in global malabsorption, which includes malabsorption of oligopeptides and AAs secondary to lack of mucosal hydrolysis of oligopeptides and defective mucosal absorption. Reduction of intestinal absorptive surface, as in short bowel syndrome (see Chapter 106 ) or jejunoileal bypass, also results in protein and AA malabsorption. Congenital defects of AA transporters on the enterocytes (e.g., Hartnup disease, lysinuric protein intolerance) can lead to selective malabsorption of a subgroup of AAs (see Chapter 102 and see Congenital Defects).
The pathophysiologic mechanisms and clinical role of carbohydrate malabsorption has been recently reviewed.
Pancreatic α-amylase is normally secreted in excess into the intestinal lumen. In mild forms of pancreatic insufficiency, carbohydrate digestion usually is at least partially preserved, but severe pancreatic insufficiency results in clinically apparent carbohydrate malabsorption and diarrhea due to decreased luminal hydrolysis of ingested starch.
The most common cause of carbohydrate malabsorption is late-onset lactose malabsorption due to decreased levels of the intestinal brush border enzyme lactase (adult-type hypolactasia, acquired primary lactase deficiency). Depending on ethnic background, lactase is present in less than 5% to greater than 90% of the adult population; its deficiency results in a selective malabsorption of lactose. Acquired malabsorption of carbohydrates occurs commonly after extensive intestinal resections, in diffuse mucosal diseases such as celiac or Crohn disease, or temporarily after self-limited GI infections (postinfection carbohydrate malabsorption). The pathophysiologic mechanisms of carbohydrate malabsorption are reduction of the intestinal mucosal surface area and reduced activity or expression of intestinal oligo- and disaccharidases or transport proteins for monosaccharides. Congenital disaccharidase deficiencies (lactase, sucrase-isomaltase, trehalase) and congenital deficiency or malfunction of transport molecules, as in congenital glucose-galactose malabsorption, can cause early onset of malabsorption of mono- or disaccharides (see Congential Defects). Intolerance of fructose is discussed in a subsequent section.
Diseases that cause malabsorption of dietary fat commonly cause malabsorption of fat-soluble vitamins because they require similar absorptive mechanisms. This is especially relevant in diseases that result in impaired micelle formation because of bile salt deficiency. Fat-soluble vitamins also are malabsorbed in diffuse diseases of the mucosal surface area, in diseases affecting chylomicron formation and transport, and in exocrine pancreatic insufficiency. Some authors have suggested that absorption of fat-soluble vitamins is less affected by exocrine pancreatic insufficiency than by small intestinal diseases with resultant steatorrhea. Genetic defects of BA conjugation may also result in malabsorption of fat-soluble vitamins.
Decreased release of dietary vitamin B 12 from food sources because of impaired pepsin and acid secretion, as in atrophic gastritis or with use of gastric acid inhibitors, usually results in only mild cobalamin malabsorption without clinical consequences. By contrast, deficiency of gastric IF secretion, as occurs in pernicious anemia (PA) or after gastric resections, or secretion of an abnormal IF, as in some congenital diseases, results in severe vitamin B 12 malabsorption with clinical consequences.
Autoimmune gastritis seen with PA is the most common cause of vitamin B 12 malabsorption (see Chapter 52 ). Cobalamin malabsorption in PA is caused by decreased IF secretion, which results from parietal cell destruction and by the production of blocking autoantibodies that inhibit binding of IF to vitamin B 12 . Mild cobalamin malabsorption may be found in patients with ZES (see Chapter 34 ) and in patients with pancreatic insufficiency (see Chapter 59 ), owing to decreased proteolytic release of vitamin B 12 from its complex with R-binding protein (see Chapter 103Chapter 103Chapter 34 ).
In SIBO (see Chapter 105 ) or helminthic infection with Diphyllobothrium latum (see Chapter 114 ), dietary cobalamin is made unavailable to the host and is, therefore, unavailable for intestinal absorption.
Diseases and conditions that affect the ileal mucosa (e.g., Crohn disease, ileal resection) lead to a reduction of specific absorptive sites for the IF-vitamin B 12 complex. Ileal resections of more than 30 cm bear the risk of clinically significant vitamin B 12 malabsorption. Imerslund-Gräsbeck syndrome, a disease of autosomal recessive inheritance due to abnormalities of the cubilin-amnionless complex, is characterized by selective ileal malabsorption of the IF-vitamin B 12 complex despite normal ileal morphology (see Chapter 103 ). Congenital diseases affecting transcobalamin II also result in malabsorption of cobalamin.
In previously healthy persons, it usually takes several years of vitamin B 12 malabsorption before cobalamin deficiency develops, because hepatic stores contain large amounts of cobalamin and the daily requirement is relatively small.
Folate malabsorption occurs with mucosal diseases that affect the proximal small intestine, such as celiac disease and Whipple disease. Folate deficiency is common in chronic alcoholuse, where it is postulated to be caused by decreased dietary intake as well as decreased intestinal absorption of folate. As discussed later, several drugs may result in impaired intestinal uptake of folate and an inherited form of selective folate malabsorption has been described. In contrast to cobalamin, body stores of folate are small relative to daily requirements; hence folate deficiency develops much faster (i.e., within weeks) than cobalamin deficiency in the setting of malabsorption. Increased serum folate levels resulting from bacterial production of tetrahydrofolate have been reported in SIBO.
Other water-soluble vitamins, such as ascorbic acid and the B-complex vitamins, are absorbed in the small intestine either by carrier-mediated transport or passive diffusion (see Chapter 103 ). Generalized malabsorption syndromes from intestinal causes impair the absorption of these vitamins, thereby leading to deficiency states. Deficiency of water-soluble vitamins also occurs with chronic alcoholuse, probably owing to decreased oral intake and reduced intestinal absorption.
Severe calcium malabsorption can occur in diseases that affect the small intestinal mucosa (e.g., celiac disease) and luminal malabsorption (e.g., exocrine pancreatic insufficiency), and has been shown to result in low-trauma fractures. In these disease states, calcium absorption is impaired directly because of the reduction of the intestinal surface area (celiac disease) and indirectly because of formation of insoluble calcium soaps with malabsorbed long-chain fatty acids (pancreatic insufficiency). Diseases that cause malabsorption of long-chain fatty acids by other mechanisms, such as bile acid deficiency, can also result in calcium malabsorption. In many of these diseases, malabsorption and deficiency of vitamin D further contribute to intestinal calcium malabsorption. Selective intestinal malabsorption of calcium (i.e., without fat malabsorption) can occur in renal disease, hypoparathyroidism, and inborn defects in formation of 1α,25-dihydroxyvitamin D or in the intestinal vitamin D receptor. Calcium malabsorption also commonly occurs after gastric resection (see “Malabsorption after Gastric Resection” [later]). Dosage of PPIs and duration of gastric acid suppression are associated with increased risk of hip fractures, presumably due in part to decreased or impaired absorption of dietary calcium, although both the magnitude and exact explanation of risk are controversial.
In many generalized malabsorptive disorders, magnesium deficiency due to magnesium malabsorption results from a reduction in mucosal absorptive surface area and luminal binding of magnesium by malabsorbed fatty acids. A congenital form of selective intestinal magnesium malabsorption has also been reported.
Iron deficiency is common in patients with gastric resection or celiac disease. Also, achlorhydria may result in iron deficiency because the absorption of iron in these patients is less than the physiologic iron losses, a phenomenon which is not overcome by the adaptive increase in duodenal iron absorption that occurs in response to iron deficiency. Reduction in the mucosal surface area of the small intestine as a result of diffuse mucosal disease, intestinal resection, or intestinal bypass can result in impaired iron absorption, potentially leading to iron deficiency. A congenital form of iron malabsorption has also been described (see Table 104.10 ). Intestinal loss of iron from chronic GI bleeding is, however, the most common GI cause of iron deficiency. Hookworm infection is the most common cause of iron deficiency worldwide (see Chapter 114 ).
| Disorder | Causative Gene | Suggested Mode of Inheritance | Malabsorbed Substrate(s) | Suggested Mechanism of Malabsorption | Clinical Features | Reference(s) |
|---|---|---|---|---|---|---|
| Malabsorption of Amino Acids | ||||||
| Hartnup disorder OMIM# 234500 |
SLC6A19 | AR | Neutral amino acids (tryptophan, leucine, methionine, phenylalanine, tyrosine, valine, ?histidine, ?lysine) | Decreased intestinal absorption of free neutral amino acids | Most patients are asymptomatic; some patients have photosensitive skin rash, intermittent ataxia, psychotic behavior, mental retardation, diarrhea | |
| Cystinuria (types A, B, AB) OMIM# 220100 |
Type A: SLC3A1 Type B: SLC7A9 |
AR (type A) and incomplete AR (type B) | Cystine and/or dibasic amino acids (lysine, ornithine, arginine) | Decreased intestinal absorption of specific free amino acids owing to a defective amino acid transporter at the brush border membrane Type A: no transport of cystine, lysine, or arginine Type B: reduced or normal cystine transport and reduced or no lysine and arginine transport |
Aminoaciduria, cystine stones in the urinary tract | |
| Lysinuric protein intolerance OMIM# 222700 |
SLC7A7 | AR | Dibasic amino acids (lysine, ornithine, arginine) | Defect of the basolateral transporter (y+LAT-1) for dibasic amino acids (also malabsorption of di- and oligopeptides) | Sparse hair, hyperammonemia, nausea, vomiting, diarrhea, protein malnutrition, failure to thrive, aversion to protein-rich food | |
| Isolated lysinuria ∗ | ? | ? | Lysine | Decreased intestinal absorption of lysine | Mental retardation, malnutrition, failure to thrive | |
| Iminoglycinuria OMIM# 242600 |
SLC6A20 SLC6A19 SLC36A2 |
AR | l-Proline | Impaired intestinal absorption of l-proline in a subgroup of subjects | Aminoaciduria; benign disorder | |
| Blue diaper syndrome ∗ OMIM# 211000 |
? | AR | Tryptophan | Intestinal tryptophan absorption defect | Blue discoloration of diapers, failure to thrive, hypercalcemia, nephrocalcinosis | |
| Methionine malabsorption syndrome ∗ (Oasthouse syndrome) OMIM# 250900 |
? | AR | Methionine | Intestinal methionine absorption defect | Mental retardation, convulsions, diarrhea, white hair, hyperpnea; urine has characteristic sweet smell of dried celery | |
| Lowe oculocerebral syndrome OMIM# 30900 |
OCRL1 | XR | Lysine, arginine | Impaired intestinal lysine and arginine absorption | Aminoaciduria, mental retardation, cataracts, rickets, choreoathetosis, renal disease | |
| Malabsorption of Carbohydrates | ||||||
| Congenital lactase deficiency OMIM# 22300 |
LCT | AR | Lactose | Permanent very low lactase activity | Diarrhea, bloating, and dehydration in the first days of life | |
| Sucrase-isomaltase deficiency OMIM# 2229000 |
SI | AR | Sucrose, starch | Sucrase activity is absent; isomaltase activity is absent or reduced; reduced maltase activity | Osmotic diarrhea after starch or sucrose ingestion; failure to thrive | , |
| Trehalase deficiency OMIM# 612119 |
TREH | AR | Trehalose | Lack of intestinal trehalase activity | Diarrhea and/or vomiting after ingesting mushrooms | |
| Glucose-galactose malabsorption OMIM# 606824 |
SLC5A1 | AR | Glucose, galactose | Defect of the brush border sodium-glucose cotransporter (SGLT1) | Neonatal onset of osmotic diarrhea, dehydration, intermittent or constant glycosuria | |
| Malabsorption of Fat | ||||||
| Abetalipoproteinemia OMIM# 200100 |
MTP | AR | Fat, fat-soluble vitamins | Defective lipoprotein assembly owing to a lack of MTP, resulting in TG accumulation in the enterocyte and no chylomicron formation | Steatorrhea, diarrhea, neurologic symptoms, retinitis pigmentosa, failure to thrive, absence of chylomicrons and VLDL in the blood, acanthocytosis | |
| Familial hypobetalipoproteinemia OMIM# 615558 |
APOB | Incomplete AD | Fat, fat-soluble vitamins | TG accumulation in the enterocyte in homozygotes owing to formation of a truncated apolipoprotein B | Homozygotes: clinical manifestations as for abetalipoproteinemia Heterozygotes: fat absorption probably normal; hypolipidemia, neurologic manifestations |
|
| Chylomicron retention disease | SAR1B | AR | Fat | Defective chylomicron formation and accumulation in the enterocyte | Steatorrhea, failure to thrive, absence of chylomicrons and reduced LDL levels in the blood; neurologic symptoms in some patients | , |
| (Anderson disease) OMIM# 246700 |
||||||
| Cholesteryl ester storage disease (Wolman disease) OMIM# 278000 |
LIPA | AR | Fat | Deficient activity of hLAL, cholesteryl ester hydrolase, causing accumulation of cholesteryl esters and TGs in various body tissues; infiltration of intestinal mucosa with foamy cells, intestinal damage | Steatorrhea, hepatosplenomegaly, abdominal distention; failure to thrive, adrenal calcifications | , |
| Malabsorption of Vitamins | ||||||
| Congenital IF deficiency (congenital pernicious anemia) OMIM# 261000 |
GIF | AR | Cobalamin (vitamin B 12 ) | Defective synthesis of IF or synthesis of an abnormal IF with either reduced affinity for cobalamin or for the ileal IF receptor, or increased susceptibility to proteolysis | Megaloblastic anemia, neurologic symptoms, delayed development | |
| Imerslund-Gräsbeck syndrome (ileal B 12 malabsorption, megaloblastic anemia type I) OMIM# 261100 |
CUBN or AMN | AR | Cobalamin (vitamin B 12 ) | Impaired ileal absorption of IF-cobalamin complex owing to defects in the cubilin-AMN complex (IF-cobalamin receptor) | Megaloblastic anemia, neurologic symptoms, proteinuria | , |
| Transcobalamin II deficiency OMIM# 275350 |
TCN2 | AR | Cobalamin (vitamin B 12 ) | Defective transport of cobalamin out of enterocytes into the portal blood owing to absence or malfunction of transcobalamin II | Vomiting, diarrhea, failure to thrive, anemia, immunodeficiency, neurologic symptoms | , |
| Hereditary folate malabsorption OMIM# 229050 |
SLC46A1 | AR | Folate | Defective folate transport across the intestinal mucosa | Megaloblastic anemia, diarrhea, neurologic symptoms | |
| Malabsorption of Minerals | ||||||
| Acrodermatitis enteropathica OMIM# 201100 |
SLC39A4 | AR | Zinc | Defective zinc absorption in the small intestine owing to a defect in the zinc transport protein (hZIP4) | Diarrhea, scaling erythematous dermatitis, alopecia, neuropsychiatric symptoms; onset after weaning | |
| Isolated magnesium malabsorption (hypomagnesemia with secondary hypocalcemia [HOMG]) OMIM# 602014 |
TRPM6 | AR | Magnesium | Selective defect in intestinal magnesium absorption | Tetany, convulsion, diarrhea, hypomagnesemia with secondary hypocalcemia | |
| Menkes disease OMIM# 309400 |
ATP7A | XR | Copper | General copper transport disorder; intestinal copper malabsorption with copper accumulation in the intestinal mucosa owing to a defective transmembrane copper-transporting ATPase (MNK) | Cerebral degeneration, diarrhea, abnormal hair, hypopigmentation, arterial rupture, thrombosis, hypothermia, bone changes | |
| Occipital horn syndrome (X-linked cutis laxa) OMIM# 304150 |
ATP7A | XR | Copper | Milder form of same defect as in Menkes disease; low levels of functional MNK | Inguinal hernias, bladder and ureteral diverticula, skin and joint laxity, chronic diarrhea, bone changes | |
| Iron-refractory iron-deficient anemia OMIM# 206200 |
TMPRSS6 | AR | Iron | Intestinal iron transport disorder | Iron-deficient anemia that is unresponsive to oral iron supplementation | |
| Hereditary selective deficiency of 1α,25(OH) 2 D (pseudo–vitamin D deficiency rickets) OMIM# 264700 |
CYP27B1 | AR | Calcium | Defective 25(OH)D 1α-hydroxylase, resulting in 1α,25(OH) 2 D deficiency and reduced intestinal calcium absorption | Bone pain, deformities and fractures, muscle weakness | |
| Hereditary generalized resistance to 1α,25(OH) 2 D (vitamin D-resistant rickets) OMIM# 277440 |
VDR | AR | Calcium | Malfunction of the vitamin D receptor owing to defective hormone binding, defective receptor translocation to nucleus, or defective receptor binding to DNA, resulting in malabsorption of calcium | Bone pain, deformities and fractures, muscle weakness, alopecia | |
| Other Defects | ||||||
| Enterokinase deficiency OMIM# 226200 |
PRSS7 | AR | Protein, fat | Defective activation of pancreatic proenzymes owing to lack of intestinal enterokinase | Diarrhea, failure to thrive, hypoproteinemia, edema, anemia | , |
| Congenital bile acid malabsorption OMIM# 613291 |
SLC10A2 | AR | Bile acids, fat | Defect of the ileal ASBT | Steatorrhea, diarrhea, failure to thrive | |
| Microvillus inclusion disease OMIM# 251850 |
MYO5B | AR | Carbohydrates, fat, cobalamin, electrolytes, water | Villus atrophy with microvillus inclusions in enterocytes, absent or shortened brush border microvilli | Severe watery diarrhea and steatorrhea requiring total parenteral nutrition | |
| Hyperinsulinism, with enteropathy and deafness OMIM# 606528 |
USH1C, ABCC8, or KCNJ11 | AR | Generalized malabsorption | Enteropathy with villus atrophy and inflammation | Hyperinsulinism, profound congenital sensorineural deafness, enteropathy, renal tubular dysfunction | |
| Immune dysregulation polyendocrinopathy and enteropathy, X-linked (IPEX) OMIM# 304790 |
FOXP3 | XR | Generalized malabsorption | Villus atrophy | Polyendocrinopathies, severe diarrhea, hemolytic anemia | |
| Enteric anendocrinosis ∗ OMIM# 610370 |
NEUROG3 | AR | Generalized malabsorption | Lack of enteroendocrine cells | Severe diarrhea, failure to thrive, type 1 diabetes mellitus | |
| Congenital proprotein convertase 1/3 deficiency OMIM# 600955 |
PCSK1 | AR | Generalized malabsorption | Lack of functional hormone production by enteroendocrine cells | Severe diarrhea, polyendocrinopathies, failure to thrive, overweight in later age | |
| Congenital tufting enteropathy OMIM# 613217 |
EpCAM | AR | Generalized malabsorption | Intestinal epithelial cell dysplasia and villus atrophy | Severe diarrhea, failure to thrive | |
| CHAPLE syndrome CD55deficiency with hyperactivation of complement, angiopathic thrombosis, and protein-losing enteropathy OMIM# 226300 |
CD55 | AR | Protein losing enteropathy, vitamin and micronutrient deficiencies | Intestinal lymphangiectasia | Anemia, growth retardation, diarrhea, abdominal pain, hypoproteinemia, thrombosis | |
Zinc, like other minerals, is malabsorbed in generalized mucosal diseases of the small intestine. A congenital selective defect of zinc absorption, acrodermatitis enteropathica, is caused by a defect in the zinc transport protein hZIP4 (see Table 104.10 ).
Generalized malabsorption can cause deficiency of copper and selenium. In Menkes disease (kinky hair disease), an inherited disorder of cellular copper transport, selective intestinal copper malabsorption results (see later). It is uncertain whether malabsorptive diseases result in deficiencies of chromium and manganese.
The colon has the capacity to absorb a limited number but wide variety of substances and nutrients, including sodium, chloride, water, oxalate, short-chain fatty acids, calcium, water-soluble vitamins (biotin, folate, pantothenic acid [B 5 ], pyridoxine [B 6 ], riboflavin [B 2 ], thiamine [B 1 ]), and vitamin K (see Chapter 103 ). Although colonic nutrient absorption plays a lesser role in health, the nutritive role of the colon in patients with severe malabsorption is clinically relevant. Colonic salvage of malabsorbed nutrients can also result in symptoms and complications such as colonic hyperabsorption of oxalate, which contributes to the formation of renal stones (see later).
The role of the colon in the pathogenesis of symptoms related to lactose malabsorption and incomplete fructose absorption (fructose malabsorption), such as bloating, abdominal cramps, and diarrhea, is unclear. A discrepancy between malabsorption, as demonstrated by hydrogen breath testing (HBT), and these intolerance symptoms have been demonstrated in adults and children.
In healthy people, between 2% and 20% of ingested starch escapes absorption in the small intestine; pancreatic insufficiency or severe intestinal disorders further increase this amount. Carbohydrates that reach the colon cannot be absorbed by the colonic mucosa but can be metabolized by colonic bacteria. Metabolism by anaerobic bacteria results in breakdown of oligosaccharides and polysaccharides to mono- and disaccharides, which are further metabolized to lactic acid, short-chain (C2 to C4) fatty acids (SCFAs; e.g., acetate, propionate, butyrate), and odorless gases (e.g., hydrogen, methane, carbon dioxide).
Studies in normal subjects have suggested that bacterial metabolism of starch to small carbohydrate moieties is a rapid process in the normal colon. The rate-limiting step in the overall conversion of polysaccharides to SCFAs appears to be the conversion of monosaccharides to SCFAs. Colonic absorption of SCFAs results in the reduction of the osmotic load and, as a result, mitigation of osmotic diarrhea. In normal subjects, more than 45 g of carbohydrates must reach the colon to cause diarrhea, and up to 80 g of carbohydrates per day can be metabolized by bacteria to SCFAs; approximately 90% of these SCFAs are absorbed by colonic mucosa ( Fig. 104.1 ). Chronic carbohydrate malabsorption causes adaptive changes in bacterial metabolic activity that result in even higher efficiency of the bacterial microbiota to digest carbohydrates, but at the expense of increased flatus production.
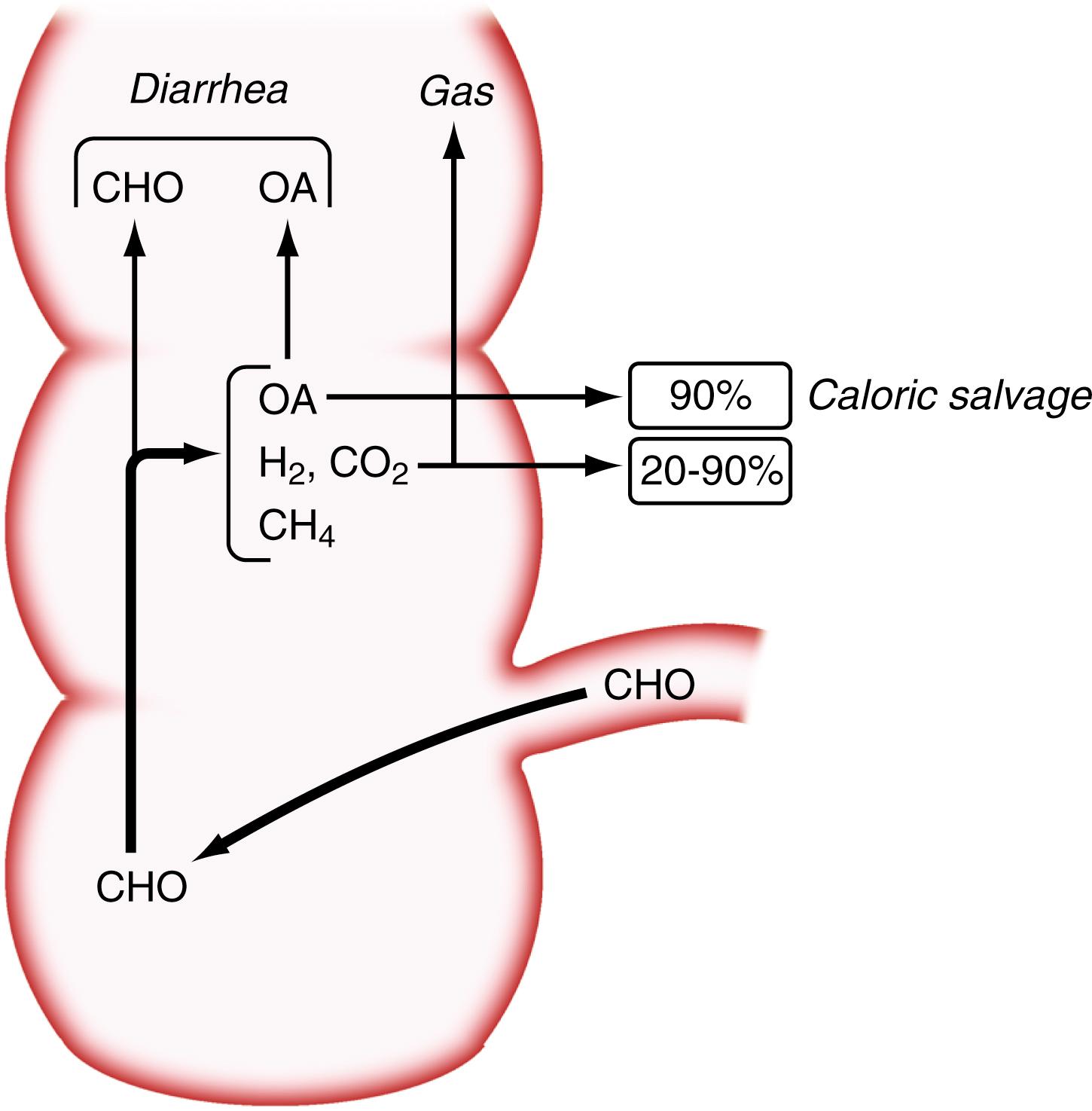
Because SCFAs have caloric values between 3.4 and 5.95 kcal/g, their colonic absorption can contribute positively to overall calorie balance. In patients with short bowel syndrome, colonic salvage of malabsorbed carbohydrates can save up to 700 to 950 kcal/day, provided a substantial part of the colon remains in continuity with the small intestine (see Chapter 106 ). Not all SCFAs are absorbed by the colon, and those not absorbed contribute to osmotic diarrhea.
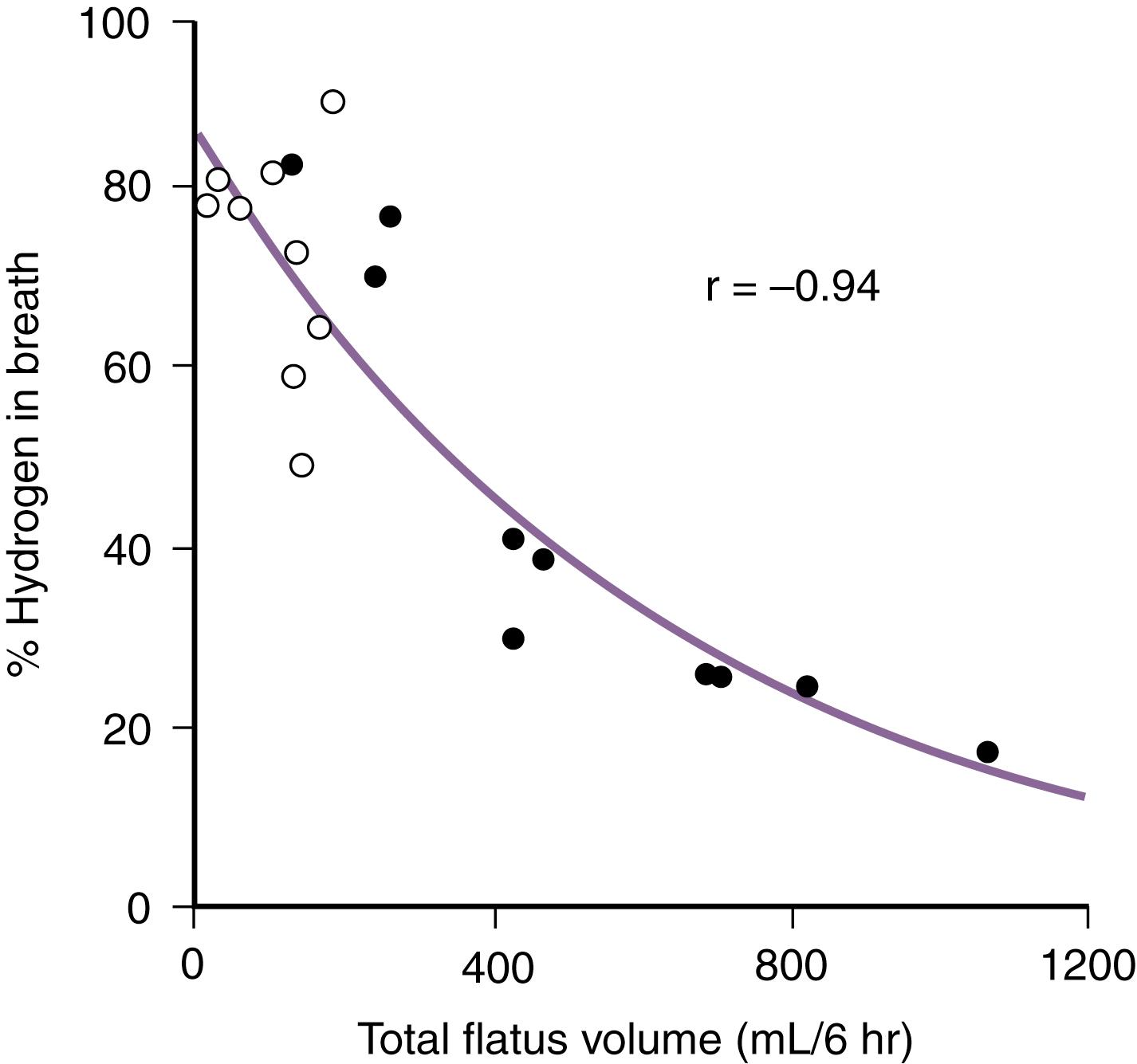
The benefits of colonic bacterial carbohydrate metabolism may be offset by side effects due to gas production (see Chapter 17 ). Up to 10-fold differences in the volume of gas produced in the colon have been observed in normal persons. The colon also can absorb gas, and if intracolonic gas volumes are low, up to 90% of the volume of intracolonic gas can be absorbed; if gas volumes are high, however, this proportion can decrease to 20% ( Fig. 104.2 ). Therefore persons who have the disadvantage of producing more gas in their colons have an additional disadvantage of absorbing a smaller fraction of the gas. Gas produced from bacterial carbohydrate metabolism is odorless. The odor of flatus is due to volatile sulfur-containing substrates that result from bacterial metabolism of protein.
Impaired colonic salvage of carbohydrates has been suggested to contribute to the diarrhea in Crohn disease and UC. Bacterial carbohydrate metabolism may be lessened by antibiotic treatment. In some patients, antibiotic-associated diarrhea may be the result of impaired colonic salvage of carbohydrates or the result of dietary fiber that can accumulate in stool because of decreased bacterial fermentation.
Long-chain triglycerides or fatty acids, which constitute most dietary fat, cannot be absorbed by the human colon. Long-chain fatty acids bind calcium in the colon, thereby increasing the amount of oxalate that is absorbed bound to sodium. Fatty acids with chain lengths longer than 12 carbons can cause diarrhea because they increase mucosal permeability and inhibit colonic absorption of fluid and electrolytes. An increase in colonic permeability due to long-chain fatty acids also may be a contributing factor for the increased colonic oxalate absorption seen in patients with steatorrhea and hyperoxaluria.
Patients with short bowel syndrome can gain caloric energy from colonic absorption of medium-chain fatty acids coming from medium-chain triglyceride supplementation if they have at least part of the colon in continuity with the remaining small intestine.
Although most unabsorbed calcium is insoluble when it reaches the terminal ileum, preservation of at least half of the colon in patients with extensive small bowel resection improves calcium absorption by about 40%, compared with calcium absorption in patients who have an ileostomy. Absorption of calcium requires solubilization of calcium salts. Bacterial metabolism of dietary fiber or incompletely absorbed carbohydrates can help solubilize calcium by causing a decrease in the pH of luminal contents in the colon. Once calcium is solubilized, it can contact the cecal mucosa, which, in the rat, has the highest calcium absorption rate per surface area of intestine. Calcium solubilization in the colon from bacterial fermentation of malabsorbed lactose can also occur in patients with lactose malabsorption; in this condition, the bioavailability of calcium from milk is greater than that from mineral water. In addition to their effect on luminal pH, the SCFAs, such as acetate and propionate, which are products of bacterial metabolism of lactose, have been shown to directly enhance calcium absorption in the human colon.
The lower parts of the GI tract do not normally contact nutrients, but upon doing so, intestinal transit time becomes prolonged. This delay in transit could contribute to compensatory mechanisms in malabsorptive diseases, although nutritional salvage by this mechanism has not been quantitated. SCFAs prolong colonic transit time and, hence, increase the contact time of liminal contents with the colonic mucosa.
Malabsorption usually is suspected on the basis of the patient’s history, signs and symptoms, or findings on routine laboratory evaluations. Malabsorption of an ingested nutrient or substrate can be confirmed by measuring its increased stool concentration, its decreased serum concentration, or its urinary excretion. Finding the cause of malabsorption often requires tests such as endoscopy with small intestinal biopsy; under certain clinical circumstances, noninvasive tests or radiologic imaging are helpful in providing a specific diagnosis.
Table 104.3 lists symptoms and signs suggestive of malabsorption, although virtually all can have causes other than malabsorption. For example, greasy stools might indicate malabsorption, but a greasy appearance also can be caused by mucus in the stool. Floating of stool in the toilet water can be due to a high stool fat content, but it can also be caused by high gas content. Nevertheless, such symptoms and signs are helpful in raising the clinician’s index of suspicion for malabsorption and in guiding the choice of which specific laboratory tests, structural evaluations, or function tests should be ordered.
| Symptom or Sign | Pathophysiologic Explanation |
|---|---|
| Cutaneous and Mucosal | |
| Acrodermatitis, scaly dermatitis | Deficiency of zinc and essential fatty acids |
| Easy bruisability, ecchymoses, petechiae | Deficiency of vitamin K, vitamin C (scurvy) |
| Edema | Protein loss or malabsorption |
| Follicular hyperkeratosis | Deficiency of vitamin A |
| Glossitis, cheilosis, stomatitis | Deficiency of vitamin B complex, vitamin B 12 , folate, or iron |
| Hyperpigmented dermatitis | Deficiency of niacin (pellagra) |
| Perifollicular hemorrhage | Malabsorption of vitamin C |
| Spiral or curly hair | Malabsorption of vitamin C |
| Thin nails with spoon-shaped deformity | Deficiency of iron |
| Gastrointestinal | |
| Abdominal distention, flatulence | Gas production from bacterial fermentation of carbohydrates in the colon, SIBO |
| Ascites | Protein loss or malabsorption |
| Diarrhea | Osmotic activity of carbohydrates or short-chain fatty acids |
| Secretory effect of bile acids and fatty acids | |
| Decreased absorptive surface | |
| Intestinal loss of conjugated bile acids: | |
|
|
|
|
|
|
| Foul-smelling flatulence or stool | Malabsorption of proteins or intestinal protein loss |
| Pain | Gaseous distention of the intestine |
| Musculoskeletal | |
| Bone pain, osteomalacia, fractures | Protein, calcium, or vitamin D deficiency; secondary hyperparathyroidism |
| Tetany, muscle weakness, paresthesias | Malabsorption of vitamin D, calcium, magnesium, and phosphate |
| Other | |
| Amenorrhea, impotence, infertility | Multifactorial (including protein malabsorption, secondary hypopituitarism, anemia) |
| Anemia | Deficiency of iron, folate, or vitamin B 12 |
| Fatigue, weakness | Calorie depletion, iron and folate deficiency, anemia |
| Growth and weight retardation, infantilism | Nutrient malabsorption in childhood and adolescence |
| Kidney stones | Increased colonic oxalate absorption |
| Night blindness, xerophthalmia | Deficiency of vitamin A |
| Neurologic symptoms, ataxia | Deficiency of vitamin B 12 , vitamin E, or folate |
| Peripheral neuropathy | Deficiency of vitamin B 12 or thiamine |
| Weight loss, hyperphagia | Nutrient malabsorption |
The current obesity epidemic has led to a changing picture of malabsorption; for example, few patients today with celiac disease are underweight at diagnosis, and some are even overweight. These patients have been reported to be less likely to present with classic features such as diarrhea or anemia, and further weight gain after dietary gluten exclusion may be a cause of increased morbidity.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here