Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Human disease is increasingly appreciated to have an underlying genetic basis. This is particularly true of neurologic disease, where genetic variants have been shown to increase risk or directly cause schizophrenia, autism spectrum disorder, and neurodegenerative diseases including Alzheimer disease, Parkinson disease, amyotrophic lateral sclerosis, and spinocerebellar ataxias. Genetic alterations that may be either inherited through the germline across multiple generations or arise de novo in the sperm or egg also contribute to development of tumors of the nervous system, and several familial tumor predisposition syndromes affect the peripheral or central nervous systems (see Chapter 22 ).
Beyond these constitutional genetic variants, it is now well recognized that cancer arises through a series of acquired (“somatic”) genetic alterations that activate oncogenes or inactivate tumor suppressor genes. Many of these somatic alterations are highly recurrent in specific tumor types (e.g. BCR-ABL1 fusions in chronic myelogenous leukemia and KRAS mutations in pancreatic adenocarcinomas) and can be diagnostically useful in distinguishing these tumor entities from their clinical or histologic mimics. Tumors of the peripheral and central nervous systems are no exception, with the majority now known to harbor one or more recurrent cytogenetic or molecular genetic alterations that can be assessed to clarify or confirm the pathologic diagnosis. Table 5.1 shows the recurrent cytogenetic and genetic alterations that have been identified to date in tumor entities routinely encountered by surgical pathologists.
| Tumor Type | Cytogenetic Alterations | Gene Alterations |
|---|---|---|
| Diffuse/anaplastic astrocytoma or secondary glioblastoma in cerebral hemispheres, adult | IDH1 p.R132 or IDH2 p.R172 mutation, ATRX mutation, TP53 mutation | |
| Diffuse astrocytoma in cerebral hemispheres, pediatric | MYB or MYBL1 rearrangement, BRAF p.V600E mutation | |
| Oligodendroglioma, adult | 1p and 19q codeletion | IDH1 p.R132 or IDH2 p.R172 mutation, TERT promoter mutation, CIC or FUBP1 mutation, NOTCH1 mutation |
| Oligodendroglioma, pediatric | FGFR1 alteration (usually tandem duplication of kinase domain) | |
| Primary glioblastoma in cerebral hemispheres, adult | Trisomy 7, monosomy 10 | TERT promoter mutation, CDKN2A deletion, PTEN mutation or deletion, EGFR amplification/mutation/rearrangement, NF1 mutation |
| High-grade astrocytoma in cerebral hemispheres, pediatric | H3F3A p.G34R/V or SETD2 mutation, ATRX mutation, TP53 mutation, PIK3CA mutation, PDGFRA amplification, MET gene fusion | |
| Diffuse midline glioma | H3F3A or HIST1H3B p.K27M mutation, ATRX mutation, ACVR1 mutation (usually co-occurring with HIST1H3B mutation), TP53 or PPM1D mutation | |
| Pilocytic astrocytoma | KIAA1549-BRAF gene fusion, less commonly other BRAF gene fusions, BRAF p.V600E mutation, FGFR1 mutation/fusion, or NTRK2 gene fusion | |
| Pleomorphic xanthoastrocytoma | BRAF p.V600E mutation, CDKN2A deletion, less commonly BRAF or RAF1 fusion | |
| Subependymal giant cell astrocytoma | TSC1 or TSC2 mutation (usually germline as part of tuberous sclerosis complex) | |
| Angiocentric glioma | MYB-QKI gene fusion | |
| Chordoid glioma of the third ventricle | PRKCA p.D463H mutation | |
| Subependymoma | Unknown | |
| Myxopapillary ependymoma | Unknown | |
| Ependymoma, spinal | Monosomy 22q | NF2 mutation |
| Ependymoma, posterior fossa | Unknown | |
| Ependymoma, supratentorial | Chromothripsis of 11q | C11orf95-RELA or C11orf95-YAP1 gene fusion |
| Choroid plexus papilloma | Unknown | |
| Choroid plexus carcinoma | TP53 mutation (often germline as part of Li-Fraumeni syndrome) | |
| Ganglioglioma | BRAF fusion or p.V600E mutation, FGFR1 or FGFR2 gene fusion | |
| Desmoplastic infantile astrocytoma and ganglioglioma | BRAF fusion or p.V600E mutation | |
| Dysplastic gangliocytoma of the cerebellum | PTEN mutation (usually germline as part of Cowden disease) | |
| Central neurocytoma | Unknown | |
| Dysembryoplastic neuroepithelial tumor | BRAF p.V600E mutation or FGFR1 mutation | |
| Papillary glioneuronal tumor | SLC44A1-PRKCA gene fusion | |
| Rosette-forming glioneuronal tumor | FGFR1 mutation, PIK3CA mutation | |
| Diffuse leptomeningeal glioneuronal tumor | Monosomy 1p | KIAA1549-BRAF gene fusion |
| Pineocytoma | Unknown | |
| Pineal parenchymal tumor of intermediate differentiation | Unknown | |
| Pineoblastoma | DICER1 mutation, RB1 mutation (usually germline as part of retinoblastoma syndrome) | |
| Medulloblastoma, WNT pathway activated | Monosomy 6 | CTNNB1 mutation, DDX3X mutation |
| Medulloblastoma, SHH pathway activated | PTCH1, SMO, or SUFU mutation, GLI2 amplification, TP53 mutation, TERT promoter mutation (only in adults) | |
| Medulloblastoma, Group 3 | Isochromosome 17q | MYC amplification |
| Medulloblastoma, Group 4 | Isochromosome 17q | MYCN amplification, CDK6 amplification |
| Embryonal tumor with multilayered rosettes | C19MC amplification, TTYH1 -C19MC fusion | |
| CNS neuroblastoma | FOXR2 rearrangement | |
| CNS high-grade neuroepithelial tumor | BCOR exon 15 internal tandem duplication, MN1 gene fusion | |
| CNS Ewing sarcoma family tumor | CIC mutation or rearrangement | |
| Atypical teratoid/rhabdoid tumor | SMARCB1 mutation or deletion, less commonly SMARCA4 mutation | |
| Meningioma (cerebral, falcine, or posterior/lateral skull base) | Monosomy 22 | NF2 mutation |
| Meningioma (anterior or medial skull base) | SMO p.L412F mutation, AKT1 p.E17K mutation, TRAF7 mutation, PIK3CA mutation | |
| Meningioma, atypical or anaplastic | Monosomy 22 plus losses of 1p, 6q, 10, 14q, 18q | NF2 mutation plus TERT promoter mutation, CDKN2A deletion |
| Meningioma, secretory | KLF4 p.K409Q and TRAF7 mutations | |
| Meningioma, clear cell | SMARCE1 mutation | |
| Meningioma, rhabdoid | BAP1 mutation | |
| Solitary fibrous tumor/hemangiopericytoma | NAB2-STAT6 gene fusion | |
| Inflammatory myofibroblastic tumor | ALK or ROS1 gene fusion | |
| Hemangioblastoma | VHL mutation | |
| Fibrous dysplasia | GNAS mutation | |
| Embryonal rhabdomyosarcoma | KRAS, NRAS, or HRAS mutation, FGFR4 mutation | |
| Alveolar rhabdomyosarcoma | PAX3-FOXO1 or PAX7-FOXO1 gene fusion | |
| Ewing sarcoma | EWSR1-FLI1 gene fusion or other EWSR1, FUS, or ERG fusion | |
| Chondrosarcoma | IDH1 p.R132 mutation, COL2A1 mutation | |
| Chordoma | SETD2 mutation or deletion, CDKN2A deletion | |
| Chordoma, poorly differentiated | SMARCB1 mutation or deletion | |
| Craniopharyngioma, adamantinomatous | CTNNB1 mutation | |
| Craniopharyngioma, papillary | BRAF p.V600E mutation | |
| Langerhans cell histiocytosis | BRAF p.V600E mutation or MAP2K1 exon 2 small in-frame deletion | |
| Erdheim-Chester disease | BRAF p.V600E mutation | |
| Primary CNS lymphoma | MYD88 mutation, CD79B mutation | |
| Meningeal melanocytoma and melanoma | GNAQ or GNA11 mutation, mutation of BAP1, EIF1AX, or SF3B1 | |
| Meningeal melanocytosis and melanomatosis | NRAS mutation, less commonly BRAF p.V600E mutation | |
| Neurofibroma | NF1 mutation | |
| Schwannoma | NF2 mutation, SMARCB1 or LZTR1 germline mutation in patients with schwannomatosis | |
| Melanotic schwannoma | PRKAR1A mutation (often germline as part of Carney complex) | |
| Perineurioma | TRAF7 mutation | |
| Malignant peripheral nerve sheath tumor | NF1 mutation, CDKN2A deletion, SUZ12 or EED mutation |
The classification scheme for CNS tumors has changed dramatically over the past decade, driven in large part by advances in genomic analysis. The 2016 WHO Classification of Tumours of the Central Nervous System has been restructured from previous editions to include an integrated diagnostic scheme that combines histologic and molecular genetic information with the goal of providing the most accurate diagnostic and prognostic information to affected patients and their caregivers. Given this new emphasis, diagnostic surgical pathologists are now required to work closely with their molecular pathology colleagues to establish integrated neuropathologic diagnoses.
This chapter is not meant as a comprehensive reference of genetic alterations in CNS tumors, nor does it serve as an exhaustive treatise on the specific intricacies of all molecular diagnostic techniques. Instead, it is intended as a primer for practicing surgical pathologists on the molecular techniques now routinely used in diagnostic laboratories, with a specific focus on benefits, limitations, and potential pitfalls in interpretation for each of the methodologies discussed. Understanding the various molecular diagnostic tests that are available and how to accurately interpret the results will help practicing pathologists in selecting the best testing to perform for each specimen they receive and achieving the best molecularly integrated diagnosis for their patients.
One recently developed method for detecting recurrent genetic alterations in brain tumors is immunohistochemistry using mutant-specific antibodies, which have been developed to recognize a specific mutant isoform of an oncoprotein. Examples that have already entered routine clinical practice include antibodies against the p.R132H mutant isoform of isocitrate dehydrogenase-1 (IDH1), the p.V600E mutant isoform of BRAF, and the p.K27M mutant isoform of the histone H3 variants, H3.3 and H3.1 ( Fig. 5.1 ). As described in detail in Chapter 6 , p.R132H mutation in the IDH1 gene is present in approximately 80% of diffuse lower grade gliomas including both astrocytomas and oligodendrogliomas and is associated with a favorable prognosis compared to those that are IDH-wildtype. Immunohistochemistry using the IDH1 R132H mutant-specific antibody is a cost-effective, sensitive, and specific screening tool for identifying those diffuse gliomas that harbor this important diagnostic and prognostic genetic alteration ( Fig. 5.1A and B ). As described in detail in Chapter 6, Chapter 7, Chapter 10, Chapter 16, Chapter 20 , the p.V600E mutation in the BRAF gene is present at high frequency in a number of CNS tumor entities including pilocytic and pilomyxoid astrocytoma, diffuse astrocytoma in children, ganglioglioma, epithelioid glioblastoma, pleomorphic xanthoastrocytoma, papillary craniopharyngioma, and metastatic melanoma. Immunohistochemistry using the BRAF V600E mutant-specific antibody is an effective assay for distinguishing those tumors that harbor this therapeutically targetable genetic alteration ( Fig. 5.1C and D ). As described in Chapter 6 , the p.K27M mutation in either the H3F3A or HIST1H3B genes, which encode the histone H3 variants H3.3 and H3.1, defines the entity “diffuse midline glioma, H3 K27M-mutant, WHO grade IV” that arises in midline structures, including the thalamus, brainstem, and spinal cord in both children and adults. Immunoreactivity for histone H3 K27M mutant protein (typically in combination with loss of H3K27me3 expression; see later discussion) is a highly specific assay for identifying those diffuse midline gliomas that harbor this genetic alteration ( Fig. 5.1E and F ).
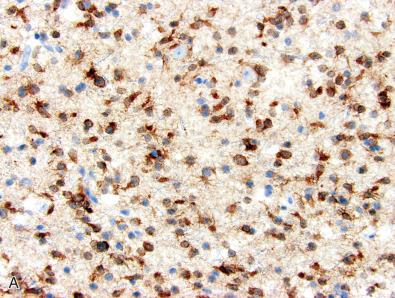
Immunohistochemistry using mutant-specific antibodies has several advantages over other methodologies for detecting single nucleotide mutations, such as Sanger sequencing. First, it can be employed on formalin-fixed, paraffin-embedded tissue by pathology laboratories around the world, including those without an affiliated molecular diagnostics laboratory capable of sequencing assays. Second, it requires very little tissue, as immunohistochemical staining can be performed and interpreted on a single tissue section, whereas molecular testing often requires multiple unstained sections to obtain sufficient DNA for analysis. Third, it does not require a high content of tumor cells within the tissue for successful evaluation. For example, individual infiltrative glioma cells can be detected by immunohistochemical staining with mutant-specific antibodies, whereas sequencing techniques may fail to detect a mutation if the relative fraction of tumor nuclei is below the detection threshold. Fourth, it allows visualization of the cells within the tissue section that express the mutant protein, which can be useful for evaluating whether a mutation is clonal and present in all of the tumor cells or if the mutation is subclonal, and only present in a subset of tumor cells.
However, there are also limitations and potential pitfalls in the use of mutant-specific antibodies for detection of single nucleotide mutations in tumor specimens. First, only a limited number of mutant-specific antibodies have been developed for diagnostic use to date relative to the large number of recurrent genetic alterations in brain tumors. Second, negative immunostaining with these antibodies does not exclude the presence of alternative mutations or other alterations in the IDH1, BRAF, and H3F3A/HIST1H3B genes. For example, a diffuse glioma that is immunonegative using antibodies against IDH1 R132H mutant protein may instead harbor one of the less common IDH1 mutations (e.g., p.R132C or p.R132S) or occasionally mutation of the equivalent p.R172 codon in IDH2 ( Fig. 5.1B ). Determining that an IDH1 R132H-immunonegative diffuse glioma is “IDH-wildtype” ultimately requires additional molecular testing via sequencing. While p.V600E mutation is by far the most common single nucleotide mutation present in BRAF in brain tumors, this gene is frequently altered instead by activating rearrangements and gene fusions in pilocytic astrocytomas, pilomyxoid astrocytomas, and occasionally pleomorphic xanthoastrocytomas and other glial neoplasms. Tumors with BRAF gene fusions or rare mutations other than p.V600E will be immunonegative using the BRAF V600E mutant-specific antibody. Relative to malignant melanomas with the BRAF p.V600E mutation, gangliogliomas and pilocytic astrocytomas that harbor this mutation express less mutant protein, often resulting in lower immunoreactivity with the BRAF V600E mutant-specific antibody, which can potentially lead to an equivocal or false negative interpretation. Also of note is that immunoreactivity in gangliogliomas may be present only in the ganglion cell component, but not the glial component in some examples, which is not completely understood (see Fig. 5.1D ). Regarding the histone H3 K27M mutant-specific antibody, tumors without H3F3A or HIST1H3B p.K27M mutations will frequently show nonspecific cytoplasmic staining of admixed macrophages and microglia but will lack the strong nuclear positivity within tumor cells that is seen in diffuse midline gliomas harboring the mutation (see Fig. 5.1F ). Also of note, a few case reports have described rare H3 K27M-mutant low-grade gliomas such as ganglioglioma and pilocytic astrocytoma, which typically have more indolent behavior, so caution is advised in using immunopositivity in isolation as a marker of the entity “diffuse midline glioma, H3 K27M-mutant, WHO grade IV.” This particular integrated diagnosis should only be made when radiographic and histologic features support a mutant-positive diffuse/infiltrative glioma centered in CNS midline structures. Finally, as with all immunohistochemical stains, false positive and false negative results can result from preanalytic variables including tissue ischemia time prior to fixation, type of fixative, length of time in fixative, antigen retrieval technique, and procedural variability of staining.
Another application of immunohistochemistry for detection of underlying genetic alterations in brain tumor specimens is using antibodies against tumor suppressor proteins that are commonly lost due to deletions or truncating mutations (e.g., nonsense, frameshift, or splice site mutations). These include ATRX, which is frequently lost in IDH-mutant diffuse astrocytomas and histone H3-mutant diffuse gliomas; SMARCB1 (also known as INI1, BAF47, and hSNF5), which is frequently lost in atypical teratoid/rhabdoid tumors and poorly differentiated chordomas; and BAP1, which is frequently lost in the more aggressive forms of rhabdoid meningioma. When evaluating immunohistochemical stains for the loss of the abovementioned tumor suppressor proteins, there should be a lack of immunoreactivity within tumor nuclei combined with intact/retained expression in non-neoplastic internal control cells, which could be admixed or adjacent endothelial cells, lymphocytes, neurons, or glial cells ( Fig. 5.2A and B ). As such, complete absence of staining in both tumor cells and admixed normal cells should be interpreted as a staining failure rather than true loss of the tumor suppressor protein ( Fig. 5.2C and D ).
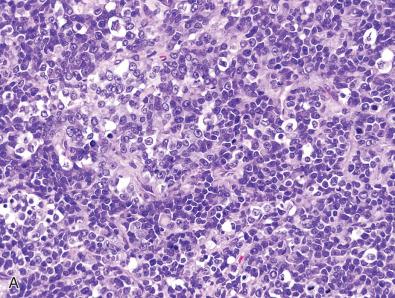
One major limitation of using immunohistochemistry to determine the genetic status of tumor suppressor genes is that these genes are not always inactivated solely by deletions or truncating mutations that result in a loss of immunoreactivity. Occasionally, tumor suppressor genes may be inactivated by missense mutation or small in-frame insertions or deletions that disrupt protein function but do not result in loss of protein expression. For example, while over 90% of ATRX and SMARCB1 alterations are deletions or truncating mutations that result in loss of protein expression, the vast majority of inactivating TP53 events are missense mutations that cause protein stabilization and overexpression of the encoded p53 protein. Because of this, diffuse gliomas and other brain tumors that harbor missense mutations in TP53 often show strong nuclear p53 staining in a large subset (typically >10%) of tumor nuclei. Therefore, this pattern of staining usually indicates a TP53 missense mutation or another alteration in the p53 regulatory pathway such as MDM2 or MDM4 amplification.
Another immunohistochemical method for assessing genetic alterations in brain tumor specimens relies on aberrant protein localization specifically in tumor cells harboring a genetic alteration that activates a signaling pathway involving the protein being studied. Two examples of this methodology that have entered routine practice in neuropathology are immunostaining for beta-catenin in medulloblastomas and craniopharyngiomas, and immunostaining for STAT6 in meningeal solitary fibrous tumors/hemangiopericytomas. One of the molecular subtypes of medulloblastoma is defined by WNT pathway activation and is associated with a favorable prognosis. WNT-activated medulloblastomas are characterized by activating somatic mutations in CTNNB, encoding the beta-catenin protein, or rarely, germline mutations in the APC tumor suppressor gene, combined with somatic loss of the remaining wildtype allele in patients with Turcot syndrome (also known as brain tumor polyposis syndrome type 2; see Chapter 22 ). In such WNT-activated medulloblastomas, beta-catenin protein is localized within the nucleus of tumor cells and can be detected by immunohistochemistry, whereas its expression is limited to the cell membrane and cytoplasm in non-WNT medulloblastomas. However, it should be noted that nuclear accumulation is often a focal finding in WNT-activated medulloblastomas, with only a subset of tumor cells (sometimes less than 1%) demonstrating nuclear beta-catenin accumulation, despite the presence of activating CTNNB1 mutation in all tumor cells. Thus, caution is advised in the use of beta-catenin immunohistochemistry to molecularly subtype small biopsies of medulloblastomas; integration with other molecular testing results such as fluorescence in situ hybridization (FISH) for monosomy 6 status or targeted sequencing is recommended for definitive molecular classification.
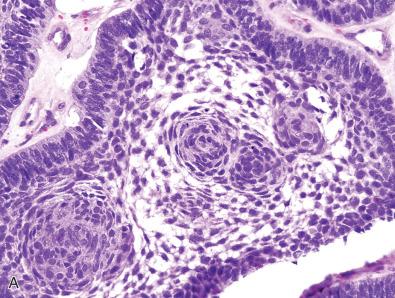
Beta-catenin immunohistochemistry may also be helpful in subtyping of craniopharyngiomas. The majority of adamantinomatous craniopharyngiomas harbor activating mutations in CTNNB1 and similarly demonstrate aberrant nuclear accumulation of beta-catenin protein. This is in contrast to the majority of papillary craniopharyngiomas, which instead harbor the activating BRAF p.V600E mutation and lack nuclear beta-catenin localization. Similar to the WNT-activated medulloblastomas, nuclear immunostaining for beta-catenin in adamantinomatous craniopharyngiomas is focal, typically within whorled clusters of squamoid cells adjacent to the peripherally palisaded epithelium ( Fig. 5.3A and B ). Additionally, the absence of nuclear beta-catenin positivity does not necessarily indicate that a craniopharyngioma is of the papillary type, as not all adamantinomatous tumors demonstrate nuclear beta-catenin protein or have detectable CTNNB1 mutations.
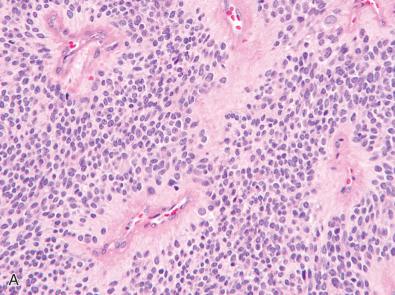
In 2013, solitary fibrous tumors and hemangiopericytomas were reported to harbor in-frame gene fusions between NAB2 and STAT6. In the absence of a fusion, the encoded STAT6 protein is normally localized in the cytoplasm, whereas the presence of a fusion leads to its aberrant accumulation within the nucleus. Immunohistochemistry for STAT6 has therefore emerged as an effective means of distinguishing solitary fibrous tumor/hemangiopericytoma from histologic mimics such as fibrous meningioma, atypical or anaplastic meningioma, and other mesenchymal neoplasms. Nevertheless, pathologists should be aware that other tumor entities have been rarely reported to show nuclear STAT6 immunopositivity or harbor NAB2-STAT6 fusions.
Another immunohistochemical method that can be used to assess for the presence or absence of clinically relevant genetic alterations in brain tumor specimens is the use of antibodies to detect proteins or post-translational modifications that are surrogate markers of underlying genetic alterations. For example, a subset of the supratentorial ependymomas in children, often those with clear cell features and branching capillaries, harbor C11orf95-RELA fusions and are associated with a poor prognosis relative to those lacking RELA fusion. The activation of the NF-kB pathway that results from RELA fusion leads to increased expression of L1 cell adhesion molecule (L1CAM), a cell surface glycoprotein. Strong, diffuse L1CAM immunoexpression in a supratentorial ependymoma indicates aberrant activation of the NF-kB pathway and is suggestive of C11orf95-RELA fusion ( Fig. 5.4A and B ). In the absence of genetic confirmation of RELA fusion status, however, a molecularly integrated diagnosis that might be appropriate in such a case is “ependymoma, L1CAM-immunopositive suggestive of RELA fusion positive variant, WHO grade II.” Other immunohistochemical surrogate markers that have been developed and entered into clinical practice include LIN28A positivity for identifying the entity “embryonal tumor with multilayered rosettes” that harbor C19MC amplification or fusion, GAB1 positivity for identifying those medulloblastomas with SHH pathway activation, and YAP1 positivity for identifying those medulloblastomas with either WNT or SHH pathway activation.
Immunohistochemical evaluation of post-translational modifications can also be diagnostically valuable as a surrogate marker for inactivation of epigenetic regulatory genes. Specifically, one of the major epigenetic regulatory modifications during cellular differentiation is trimethylation at lysine-27 on the tail of histone H3 (H3K27me3), a modification that is established by the polycomb repressive complex (PRC2). Most malignant peripheral nerve sheath tumors (MPNST) harbor inactivating mutations in the SUZ12 or EED genes that encode subunits of the PRC2 complex, leading to complete loss of H3K27me3 in tumor cells (i.e., staining is present only in non-neoplastic cells, providing a positive internal control). Loss of H3K27me3 immunoreactivity can therefore be a valuable diagnostic marker of MPNST, as other high-grade sarcomas such as rhabdomyosarcoma and leiomyosarcoma rarely show H3K27me3 loss or harbor mutations in genes encoding the PRC2 complex. Nevertheless, caution is warranted with this marker since a mosaic pattern or partial loss is considerably less specific. Complete loss of H3K27me3 immunostaining has also been suggested as a surrogate marker for the PFA molecular subgroup of posterior fossa ependymomas in children, in order to distinguish it from the less aggressive PFB molecular subgroup, even though H3K27me3 loss has not been associated with a specific underlying genetic alteration in these ependymomas to date ( Fig. 5.4C and D ). Lastly, complete loss of expression often accompanies H3 K27M mutant protein expression in diffuse midline gliomas.
While immunohistochemical surrogate markers can provide diagnostically and prognostically useful information, they are not always highly sensitive or specific markers for the underlying genetic alteration. For example, not all supratentorial ependymomas with RELA fusion demonstrate L1CAM immunoreactivity and rare ependymomas without RELA fusions have been found to have L1CAM expression, presumably because the NF-kB pathway is activated by other mechanisms. Therefore, genetic confirmation of the underlying genetic alteration is recommended when possible.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here