Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
By the production of erythropoietin (EPO) the kidneys participate in the regulation of red cell and platelet formation. This chapter reviews the present knowledge about the structure, the receptors, the functions and the regulation of production of these hormones. The two hormones show similarities with regard to structure and receptor function but strongly differ with regard to the regulation of their production. Whilst the production of EPO in the kidney is tightly regulated by the interstitial oxygen tension is the synthesis of TPO mainly a constitutive one. Therefore the control of EPO production in vivo, on the level of the kidney and on the cellular level is considered in more detail. In addition, dysregulation of EPO production as a reason of anemia and the use of EPO or EPO stimulating agents for the treatment of anemia are considered as well.
Keywords
erythropoietin, thrombopoietin, red cells, platelets, anemia, hypoxia inducible factor, prolyl-hydroxylase
During ontogeny, hematopoiesis—comprising erythropoiesis, granulocytopoiesis, and thrombopoiesis—is initiated in the blood islands of the yolk sac before the development of the kidneys. Later during gestation, at a time when hematopoiesis is fully established in the bone marrow, the kidney starts to play an important role in the control of erythro- and thrombopoiesis. This control is achieved through renal production of the hematopoietic growth factors erythropoietin and thrombopoietin. Thus, in addition to its role in waste excretion and water and electrolyte homeostasis, the kidney is also important in tissue oxygenation and coagulation.
With the exception of the growth period, the circulating red blood cell (RBC) mass, and hence the oxygen-carrying capacity of the blood, is normally fairly constant. Since the erythron is a continuously regenerating organ, a daily production of 20 ml of red blood cells is required in human adults to compensate for the physiologic demise of 120-day-old erythrocytes. In addition, when increased blood loss occurs or the oxygen saturation of hemoglobin falls, the bone marrow is capable of increasing this normal production rate of red blood cells three- to five-fold within a few days, and even up to seven-fold under chronic conditions. Similarly, the number of circulating platelets is kept constant (although in a wider range than red blood cells), and any decrease of platelet number results in activation of thrombocytopoiesis.
The predominant and essential regulators of red cell and platelet formation, erythropoietin and thrombopoietin are true hormones that are produced outside the bone marrow, and mainly in the kidney and in the liver.
This chapter is designed to summarize current knowledge about the specific role of the kidney in the humoral control of hematopoiesis both under physiologic and pathophysiologic conditions.
More than hundred years ago after the relationship between high altitude and inadequate tissue oxygenation was realized, it was postulated that hypoxia accounts for the adaptive increase in red cell mass that occurs during altitude exposure. While it was primarily believed that low oxygen pressure directly stimulates the bone marrow, the existence of a plasma factor regulating erythropoiesis was inferred from experiments in the early twentieth century, and the term “erythropoietin” was coined to describe this putative factor. However, it took another 50 years until convincing evidence was obtained for humoral oxygen-dependent control of erythropoiesis. At that time, Erslev was able to stimulate erythropoiesis in rabbits temporarily through the injection of plasma from anemic donor animals, and Reissman observed increased red cell formation in parabiotic rats kept at normal levels of oxygen when their partners were maintained in a hypoxic atmosphere. Surprisingly, it was found that—in addition to insufficient oxygen supply—the transition metal cobalt is also capable of inducing erythropoietin formation in vivo . Systemic organ ablation analysis then revealed that the capability of the organism to elaborate erythropoietin in response to anemia or to cobalt is markedly attenuated in bilaterally nephrectomized rats. This was the starting point to consider a direct regulatory role of the kidney for erythropoiesis and a complement to the observation made much earlier that patients with kidney disease are usually anemic. Nevertheless, the knowledge about erythropoietin remained rather limited, and erythropoietin was considered as an “elusive” hormone. This becomes understandable because the plasma concentration in healthy mammals is around a few femtomoles per milliliter. Continuous efforts to purify erythropoietin from the plasma of anemic animals have therefore not been successful, but provided very helpful information about the physicochemical properties of erythropoietin. Using the information that erythropoietin is a heat-stable acidic glycoprotein, Miyake et al. then succeeded to purify human erythropoietin to apparent homogeneity from about 2500 liters of urine of patients suffering from severe aplastic anemia and obtained a few milligrams of the hormone. These small amounts allowed them to determine critical amino acid sequences, and with the help of recombinant DNA techniques, two groups independently succeeded to clone the human erythropoietin gene in 1985. The knowledge of the genomic and complementary DNA sequence of erythropoietin and the availability of pure recombinant erythropoietin initiated a burst of activity leading to a greatly improved knowledge base about erythropoietin itself—action, regulation, and clinical use—and overall this work confirmed the important role of the kidneys as the primary site of erythropoietin production.
To the present, evidence for the existence of erythropoietin has been obtained for mammals. Whether erythropoietin also exists in lower organisms or in all vertebrates is not yet known. Evidence for erythropoietin genes was also reported for teleosts including fugu, zebra fish and trout. Interestingly, this gene was found to be mainly expressed in the kidney, which is also an erythropoietic organ in these teleost fish. Recently also erythropoietin of Xenopous laevis has been characterized. The amino acid sequences of erythropoietin in mammals show a high degree of interspecies similarity. First, erythropoietin is generally synthesized as pre-erythropoietin that contains a typical endoplasmatic leader sequence of 25–27 amino acid residues that is cleaved off during intracellular processing. Second, the size of native erythropoietin is similar if not identical among species and comprises 165–167 amino acids. It was shown that the carboxy-terminal arginine of human erythropoietin is deleted from the molecule, so that the 165 amino acids may be the species-independent length of circulating erythropoietin. Third, the overall structure of the molecule is highly conserved. For example, out of 15 amino acid replacements between human and monkey and 41 replacements between human and mouse, roughly 50% are conservative. The high sequence conservation also explains the interspecies cross-reactivity of erythropoietin. From bioassays for erythropoietin, it is known that human, rat, monkey, dog, and rabbit erythropoietin are active in the mouse, and human erythropoietin also stimulates erythropoiesis in ewes, cats, and dogs. In keeping with the high degree of conservation, erythropoietin also shows no homology with other known proteins, with the exception only of thrombopoietin.
Erythropoietin has a four-helical bundle structure with two disulfide bonds ( Fig. 92.1 ). One disulfide bridge between cys 7 and cys 161 links the N-terminus and the carboxy-terminus of the molecule together, generating a long loop. This disulfide bridge is fully conserved in mammals and it appears therefore as if formation of the long loop is essential for the biologic activity of erythropoietin.
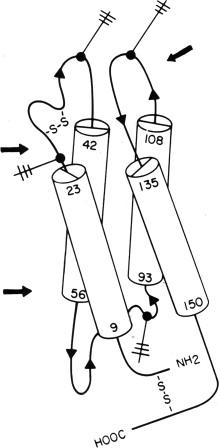
Erythropoietin is heavily glycosylated and different carbohydrate chains attached to the molecule contribute more than 40% to the total molecular weight of 30,400 Daltons. The protein backbone contains three highly conserved sites of N-glycosylation, to which sugar side chains are attached (Asn 24, 38, 83). In addition, human but not mouse erythropoietin also contains a serine (Ser 126) as a possible O-linked glycosylation site, which bears a small oligosaccharide chain.
The oligosaccharide structures of recombinant human and native urinary erythropoietin have been determined and were found to be rather similar. On average, one molecule of recombinant human erythropoietin contains 3 fructose, 10 mannose, 13 galactose, 16 N-acetylglucose, and 13 sialic acid molecules. The major component of the O-linked sugar is a disialyl chain. The N-linked side chains are predominantly organized as tetra-antennaries (about 80%) and to a minor extent only as tri- (15%) or bi-antennaries (1–6%). All of these saccharides are sialylated, although to a different extent. The distribution of different sugar chains varies at each glycosylation site. Chains at position Asn-38 are more heavily sialylated and less frequently of biantennary structure than those at Asn-24. Oligosaccharides at position Asn-83 are relatively homogenous tetra-antennary chains without polylactosaminyl repeats. The glycosylation of erythropoietin results in slight heterogeneity of erythropoietin molecules. Interestingly, clear differences were found in the distribution of oligosaccharide components of urinary erythropoietin among individuals.
The carbohydrates appear to be of considerable importance for the biologic activity of the hormone. Removal of the terminal sialic acids also increases the biologic activity of erythropoietin in vitro about two- to three-fold. The carbohydrate moieties of erythropoietin therefore appear not to be essential for binding of erythropoietin to its receptor or the subsequent cellular signaling and may even inhibit both steps. However, in vivo the carbohydrate moiety is essential for the availability of erythropoietin for its target structures. Full deglycosylation abolishes the in vivo activity of erythropoietin, and removal of terminal sialic acids inactivates the hormone by shortening its biologic half-life in the circulation from about six hours to a few minutes. The liver contains asialo-glycoprotein receptors that clear glycoproteins with free galactosyl residues from the circulation, and clearance through these receptors presumably causes the dramatic shortening of the biologic half-life of asialo-erythropoietin in vivo . How and where this modified erythropoietin and the fully deglycosylated erythropoietin are cleared from the circulation is not yet known.
Hematopoiesis : Erythropoietin is absolutely necessary for erythropoiesis, particularly for the later stages of erythroid differentiation, and therefore is essential for life. Mice with disrupted erythropoietin or erythropoietin receptor genes die from anemia at an embryonic stage of 12–13 days after fertilization because they cannot develop definitive erythropoiesis.
Erythropoiesis appears as a hierarchy of cell divisions and cell differentiations starting from the pluripotent stem and ending with the erythrocyte as a highly differentiated and specialized cell ( Fig. 92.2 ). The pluripotent stem cell, which has the capability for self-renewal, feeds into three hematopoietic lineages, namely the megakaryocytic pathway, the granulocyte-macrophage pathway, and the erythroid pathway. For the erythroid lineage cells can be identified morphologically by specific markers at the state of the proerythroblast, which develops into the basophilic erythroblast and begins to produce hemoglobin. Downstream of the erythroblast stages, the erythroid lineage is not regulated significantly by erythropoietin, nor does the commitment of pluripotent stem cells into the erythroid lineage require erythropoietin. The self-renewal of committed early erythroid progenitors is induced by the receptor tyrosine kinase c-ErbB, whereas the downstream initiation of the erythroid differentiation program is then regulated by c-Kit and erythropoietin receptors. Thus, the main action of erythropoietin for erythropoiesis occurs on erythroid progenitors at stages between early committed erythroid precursors on the one side and the proerythroblasts on the other. Information about growth and differentiation of these cells has predominantly been obtained with cell cultures of hemopoietic cells. From those experiments, the existence of a rather early erythroid progenitor was inferred that was termed burst-forming unit erythroid (BFU-E), which has an abundance of about 0.01% among the nucleated cells of the bone marrow. Within 1 week of cell culture, a burst containing multiple subcolonies in clusters with up to 104 cells develops from one BFU-E. Hypertransfusion and acute erythropoietic stress have only a transient effect on BFU-E, suggesting that this progenitor cell is not dependent on ambient levels of erythropoietin for maintenance of its numbers. In vitro , the early divisions of this progenitor and the development of erythroid bursts essentially require interleukins, in particular IL-3, but are independent on erythropoietin. Final maturation and hemoglobinization of the cells of the burst are erythropoietin dependent. Hemoglobin synthesis begins at the stage of the proerythroblast and the cell population at this stage is considered as the main target for erythropoietin. In vitro , these cells are reflected by the colony-forming unit erythroid (CFU-E). In the normal bone marrow, about 0.1–0.2% of nucleated cells are CFU-E. The cells have limited proliferative potential, probably not more than six or seven cell divisions. Thus, CFU-E-derived colonies generally contain 60 to 120 hemoglobinized cells. Colony formation in vitro is essentially dependent on erythropoietin, and is positively modulated by insulin-like growth factor I. CFU-E has the highest density of erythropoietin receptors within the normal erythroid precursor population, and is very sensitive toward erythropoietin. In vivo , the abundance of CFU-E is dependent on erythropoietin, such that the number of CFU-E increases during bleeding or hemolytic anemia and also in response to erythropoietin injection, whereas hypertransfusion-induced polycythemia results in a 70–80% reduction in the number of CFU-E in marrow and spleen. In summary, erythropoietin acts on erythropoiesis mainly by regulating the pool size of CFU-E, which then undergoes terminal differentiation into erythrocytes.
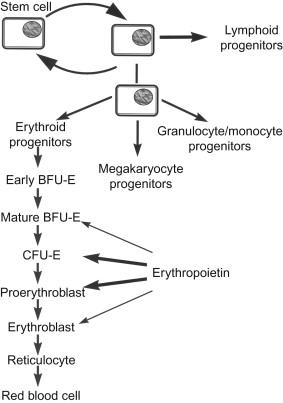
Expansion and maturation of the CFU-E pool implies three major effects of erythropoietin. First, erythropoietin increases the survival rate of erythroid progenitors and in line with this assumption evidence has been elaborated that erythropoietin prevents apoptosis in erythroid precursors. Second, erythropoietin actively induces mitosis in erythroid precursors with a preceding stimulation of RNA polymerase activity and subsequent DNA synthesis and cell division. Third, erythropoietin initiates differentiating events such as the expression of globin chains, hemoglobin synthesis, transferrin receptor synthesis, and the synthesis of integral erythrocyte membrane proteins. After induction by erythropoietin hemoglobin synthesis persists and becomes independent of the presence of erythropoietin. High doses of erythropoietin also stimulate platelet formation and erythropoietin binding sites have been reported on megakaryocytes. In view of the structural similarity between erythropoietin and thrombopoietin, one could imagine that this effect is at least in part due to cross-activation of the thrombopoietin receptor by erythropoietin.
Effects of erythropoietin unrelated to hematopoiesis : A steadily increasing number of reports clearly indicates that erythropoietin is involved in a number of effects outside the hemopoietic tissues, such as on the vasculature, the brain, the myocardium and else. There it may stimulate proliferation and inhibit apoptosis. For more detailed information, the reader is referred to recent reviews on this topic.
Distribution : The biologic effect of erythropoietin is mediated by specific binding sites at the surface of its target cells. Erythropoietin binding sites have been identified on erythroid precursors, on megakaryocytes, endothelial cells, in distinct areas of the brain, cultured neurons, and placenta.
The expression of the erythropoietin receptor is crucial for definitive erythropoiesis in vivo , since disruption of erythropoietin receptor gene leads to death around embryonic day 13. Primitive erythropoiesis in the yolk sac, however, proceeds in the absence of erythropoietin receptors. Erythropoietin receptors are also not required for erythroid lineage commitment nor for the proliferation and differentiation of BFU-E to CFU-E, but they are crucial in vivo for the proliferation and survival of CFU-E progenitors and their irreversible terminal differentiation. In line with this functional dependence, erythropoietin receptors are mainly found on erythroid precursors of the CFU-E stage. Nonetheless, compared with other cell surface receptors, CFU-E express a comparatively small number of erythropoietin receptors with 800–1200 binding sites per cell. The binding sites appear to occur with two distinct affinities, a high-affinity one with a dissociation constant below 100 pmol/liter and a low-affinity one with a dissociation constant higher than 300 pmol/liter. Binding sites with both affinities can be found on the same cell, and it is thought that the erythropoietic activity is mainly mediated via the high affinity binding site, because cells lacking these binding sites fail to differentiate in response to erythropoietin.
Erythropoietin receptor gene and protein: Erythropoietin binds and activates a high-affinity receptor (EpoR) present on the surface of immature erythroid cells, exerting four known effects on erythroid progenitors, namely maintenance of viability, promotion of cell division, increase of hemoglobin synthesis, and fostering of morphological maturation. No other cytokine receptors can replace the erythropoietin receptor, because mice homozygous for deletions of the EPO or EPO receptor genes die at embryonic day 12.5 owing to severe anemia. In these mice, erythropoiesis progresses through the BFU-E stage, but terminates at the colony forming unit-erythroid (CFU-E) and erythrocytes are not formed, demonstrating an absolute requirement of the EPO receptor for later stages of red blood cell development. The EPO receptor belongs to the cytokine receptor superfamily. Cytokine receptor signal transduction is mediated by cytoplasmic protein tyrosine kinases of the JAK family, found associated with individual receptor subunits ( Fig. 92.3 ).
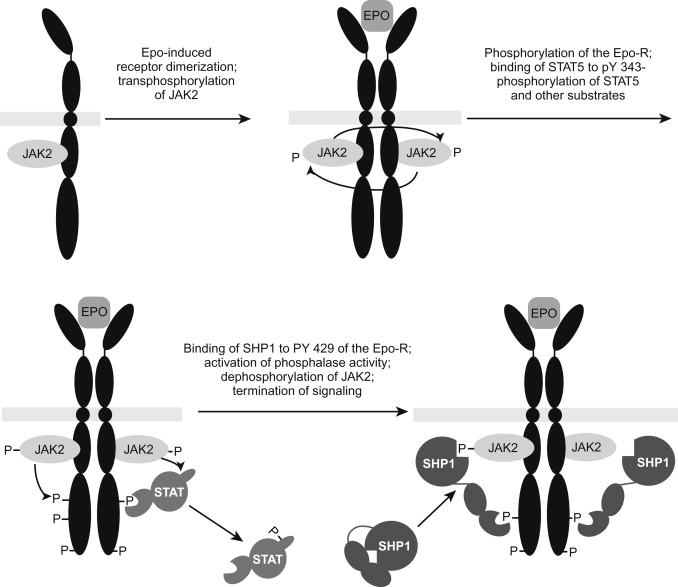
The erythropoietin receptor genes exist as single copies and encompass eight exons. In all species the cDNA encode with a high degree of interspecies homology a 507–amino acid protein of 55 kDa, which is modified by glycosylation and phosphorylation to 72–78 kDa.
The EPO receptor does not have an intrinsic enzymatic activity; however, numerous signaling proteins and the receptor itself are phosphorylated as a result of EPO stimulation. Like other members of the cytokine receptor family, such as receptors for growth hormone, prolactin, granulocyte colony-stimulating factor (G-CSF) and thrombopoietin, EpoR forms ligand-induced homodimers. EPO binding results in homodimerization of EpoR chains, which is critical for Jak2 (associated with the EpoR cytoplasmic tail) activation and the initiation of intracellular signaling. JAK2 is rapidly phosphorylated in response to EPO stimulation. JAK2 is the primary kinase responsible for phosphorylation of the EPO receptor and is essential for erythropoiesis.
Erythropoietin receptor modifications : Mutations in the extracellular domain of the EpoR within the dimer interface renders the receptor constitutively active as a result of the formation of an intermolecular disulfide bond. The carboxyterminal end of the erythropoietin receptor appears to contain an inhibitory signaling element. A hereditary truncation of the erythropoietin receptor at the COOH end has been discovered that is associated with increased affinity of the receptor and with erythrocytosis. Similar to other members of the hematopoietin receptor family such as interleukins or thrombopoietin, a naturally occurring soluble form of the membrane-bound receptor for erythropoietin has been found in the serum. It has a molecular mass of 34 kD, is related to the extracellular domain of the erythropoietin receptor, and increased concentrations are found with enhanced erythropoiesis. The physiological role of these soluble receptors and potential modes of action, however, are not yet understood.
Sites of erythropoietin production : During fetal life the liver appears to be the predominant site of erythropoietin production. This has been shown for sheep and rats, and the relevance of the liver for erythropoietin formation in the human fetus may be assumed from the fact that the cDNA for human erythropoietin has been cloned from human fetal liver. Investigations in fetal sheep revealed that the kidneys are also significant production sites for erythropoietin, particularly during the first half of gestation. In sheep and rat, a predominance of hepatic erythropoietin formation was found to last beyond birth, whereas in mice the majority of erythropoietin mRNA has been observed in the kidneys from mid-gestation on. An analysis performed in rats revealed that the kidney steadily increases its capacity for erythropoietin mRNA expression from birth on until adulthood, whereas the hepatic erythropoietin mRNA concentration changes less dramatically postpartum and even decreases when adulthood has been reached.
The kidney overtakes hepatic erythropoietin mRNA three weeks after birth. Although species differences in the timing of the switch and the degree of contribution of the liver to total body EPO mRNA content exist, the switch is undoubtedly found in humans as well.
The reasons for the postnatal increase of renal erythropoietin formation may be related to the fact that in some species the kidney is not fully mature at birth. Once the kidney has gained its full function for waste excretion and salt and water homeostasis, the maximal capacity for erythropoietin has been reached. Apparently changes of organ oxygenation are not a major trigger for the switch from liver to kidney. Based on experiments in anephric animals, the adult liver was generally assumed to contribute no more than about 15% to erythropoietin production. However, this experimental approach may underestimate the hepatic contribution in intact organisms, because hepatic erythropoietin mRNA accumulation was found to be reduced after bilateral nephrectomy. In intact adult rats erythropoietin mRNA amounts to about 30–40% of the total under conditions of severe hypoxic stimulation. However, the hepatic contribution depends on the severity of the stimulus applied and is less significant, that is, below 20% of the total under mild hypoxia. This suggests that the liver is less sensitive in terms of hypoxia-induced erythropoietin formation than the kidneys, and it might help to explain why the liver, despite its large potential for erythropoietin mRNA expression, does not compensate for failure of the renal production site.
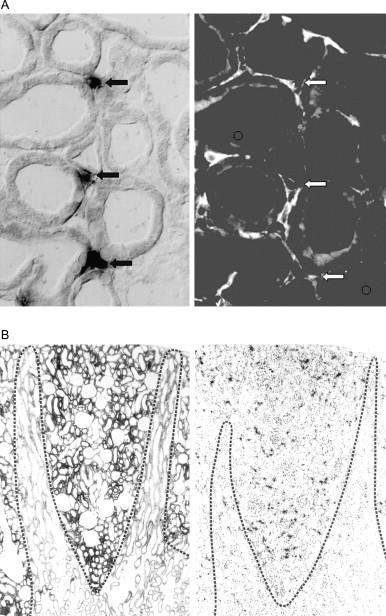
Cellular production sites in the kidney : In contrast to a number of other hormones, erythropoietin does not appear to be stored in the cells producing it. Consequently, almost no erythropoietin can be extracted from the liver and kidney of normoxic animals. Attempts to localize erythropoietin by means of immunohistochemistry in normoxic and hypoxic animals have revealed inconclusive results. In situ hybridization studies localized erythropoietin mRNA to peritubular cells in the renal cortex. Likely, peritubular interstitial fibroblasts are production sites for erythropoietin. In this context colocalization was demonstrated for erythropoietin mRNA and the enzyme 5′-ectonucleotidase, which is expressed on peritubular fibroblasts, but not on endothelial cells ( Fig. 92.4 ). The same conclusion about the peritubular fibroblast as production site for erythropoietin has been reached in an elegant approach of marker gene expression in mice bearing a transgene of erythropoietin/SV-40 T. In response to anemia or hypoxia the expression of the EPO-TAg gene was induced, and SV-40 T antigen accumulated in nuclei of peritubular cortical fibroblasts expressing 5′-nucleotidase, but not in other peritubular cell types. Erythropoietin gene expression has been demonstrated in interstitial, nonendothelial cells in normal human kidneys, as well as in the walls of renal cysts of patients with polycystic kidney disease. Similarly, renal erythropoietin has been localized in cortical interstitial cells in monkey and sheep. Although fibroblasts are widely distributed in the kidney both in the cortex and in the medulla, erythropoietin production appears to be confined to a special subset of fibroblasts in particular locations. Thus, cells expressing erythropoietin mRNA are generally not found in the inner renal medulla. At least in rats, they are not present in the outer medulla, and within the renal cortex they have almost exclusively been found in the cortical labyrinths created by the convoluted tubules, and not in the medullary rays. Even under severe hypoxia, however, only a minority of interstitial fibroblasts in the peritubular cortical labyrinth express erythropoietin mRNA.
In addition, there are reports about enhanced expression of erythropoietin mRNA in human hypernephromas, and in one tumor, erythropoietin mRNA was clearly localized to epithelial tumor cells, indicating that tubular cells may acquire the ability to produce erythropoietin with malignant transformation.
Cellular production sites in the liver : Within the liver at least two different cell types can express the erythropoietin gene. In situ hybridization in anemic rats demonstrated erythropoietin mRNA in hepatocytes, as well as in an interstitial cell type. Similarly, in severely anemic mice transfected with the human erythropoietin gene, human erythropoietin mRNA was mainly localized to hepatocytes and to a lesser extent to an interstitial cell type, while endogenous mouse erythropoietin mRNA was not demonstrable in these animals. That hepatocytes are a site of erythropoietin production was further confirmed by mRNA analysis in cells isolated from hypoxic rat livers, and the observation that in freshly isolated hepatocytes from normoxic animals erythropoietin mRNA expression can be stimulated by hypoxia in vitro .
In contrast, the abovementioned mice bearing a erythropoietin SV-40 T gene construct were found to express the T40 antigen in Ito cells of the liver following stimulation by hypoxia or anemia, indicating that these perisinusoidal cells represent the nonparenchymal cell population that produces erythropoietin in the liver. Moreover, in isolated Ito cells in culture, erythropoietin mRNA expression can be induced by hypoxia. Interestingly, the Ito cells of the liver share similarities with the renal peritubular cells expressing the erythropoietin gene. In particular, the Ito cells, like the peritubular fibroblasts in the renal cortex, express 5′-ectonucleotidase.
Other cellular production sites : Apart from liver and kidneys, small amounts of erythropoietin mRNA have also been observed in other organs such as the lung, spleen, heart, and bone marrow. In these organs, erythropoietin mRNA expression is stimulated by anemia or hypoxia and thus apparently regulated like in the kidney and the liver (see below). The physiological relevance of erythropoietin formation in organs other than liver and kidney is probably a para/autocrine one such as in the bone marrow. For the heart, EPO appears to be necessary for proper cardiac morphogenesis, and might protect the myocardium from ischemia reperfusion injury.
EPO expression in the reproductive tract and in the brain has been extensively considered. EPO is expressed in the central nervous system in the human fetus early in gestation, suggesting a central role for normal brain development. In addition, EPO has been found to be neuroprotective, particularly in situations of insufficient oxygen supply to the brain. For the female reproductive tract, regulated expression of EPO in the uterus and the oviduct of the mouse has been found. The human endometrium expresses EPO throughout the menstrual cycle, with higher levels in the secretory than in the proliferative phase. Along the same lines, estrogens stimulate EPO expression in the mouse oviduct and uterus, but neither in the brain nor in the kidney. Functionally, EPO appears to play an important role for endometrial angiogenesis and thus reproductive function. EPO mRNA expression has been found in the human, murine, and ovine placenta, as well as on both the maternal and the fetal sides. In addition, EPO exerts a trophic effect on the fetal gastrointestinal tract when taken up enterally from the amniotic fluid.
Physiological determinants regulating erythropoietin production : Numerous experimental and clinical studies have developed the concept that circulating erythropoietin levels in turn are related to a balance of tissue oxygen supply and oxygen need. As a consequence, erythropoietin appears to carefully adapt red blood cell formation to meet the oxygen requirements of the organism. Comparing the role of both oxygen supply and demand for erythropoietin concentrations, the influence of oxygen supply appears to be of major importance. It has been established that reductions of oxygen supply either by anemia or by a fall of the arterial oxygen tension are by far the most powerful stimuli for elevations of the circulating erythropoietin concentration. Apart from these classic stimuli, it was recognized early on that the pharmacological application of certain transition metals, in particular cobalt induces significant and reproducible elevations of circulating erythropoietin levels.
Effect of arterial oxygen tension : Both hypobaric normobaric hypoxia stimulate erythropoietin levels to a similar extent indicating that, it is a decline in alveolar oxygen tension and consequently a fall in arteriolar oxygen tension that stimulate erythropoietin levels. Following a sudden fall of inspiratory, and subsequently, arterial oxygen tension plasma, erythropoietin levels start to rise after 60–90 minutes and then increase approximately linearly. The slope of this increase is dependent on the severity of hypoxia, a maximum value is reached between 12 and 24 hours in rodents and within 48 hours in humans; thereafter, erythropoietin declines to a steady-state level that is well below peak concentration, but still elevated above baseline and inversely related to the severity of hypoxia ( Fig. 92.5A ). Since the decline of plasma erythropoietin levels, which occurs long before an increase in red cell mass, proceeds in parallel with decreases in renal and hepatic erythropoietin mRNA levels, it is reasonable to assume that the production of erythropoietin is downregulated during prolonged hypoxia. It has been excluded in this context that the downregulation of erythropoietin production is causally related to erythropoietin itself in the sense of a negative feedback, and it is likely therefore that a desensitization of the cellular oxygen sensing mechanism occurs during continuous hypoxia (see below). Once plasma erythropoietin concentrations have moved out of the normal range, they are inversely related to the alveolar or arterial oxygen tension at any time after onset of hypoxia.
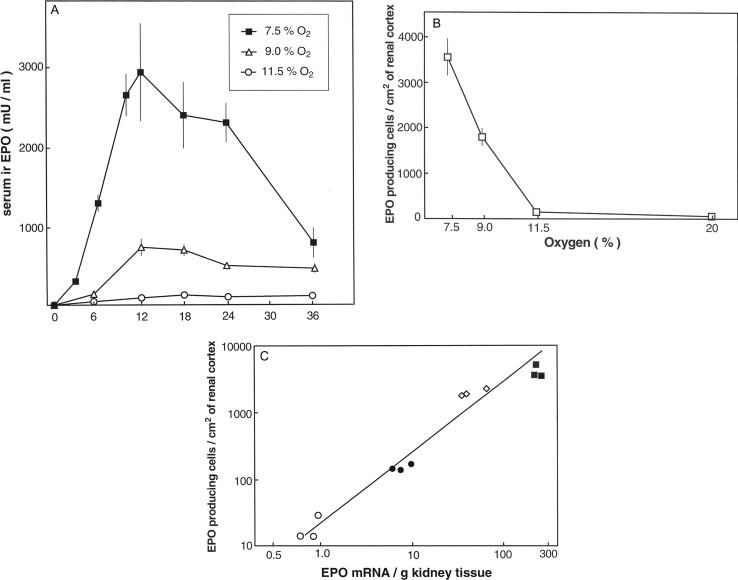
The influence of oxygen tension on plasma erythropoietin concentrations is quite characteristic in the way that a lowering of the oxygen tension to about half of the normal value only exerts a rather small effect on circulating erythropoietin levels. A fall of inspiratory oxygen tensions below this threshold then induces an almost exponential increase of plasma erythropoietin concentration in humans and in laboratory animals. The arterial oxygen tension, however, does not appear to directly influence erythropoietin levels, since the stimulation of plasma erythropoietin by inspiratory hypoxia is attenuated by polycythemia. In conclusion, it appears therefore that the effect of the oxygen tension on erythropoietin concentrations is indirectly mediated by a process essentially involving the number of red blood cells.
Effect of blood oxygen-carrying capacity : The relevance of the red cell count for the plasma erythropoietin concentration is known based on numerous observations that anemias of various etiology (with the exception of renal anemia) are associated with elevated plasma erythropoietin levels, while states of primary polycythemia move in parallel with subnormal erythropoietin concentrations. In principle, anemia could lead to an elevation of plasma erythropoietin levels by reduction of the oxygen-carrying capacity of the blood, as well as by the fall of the number of circulating red blood cells per se. This latter possibility is not trivial since, for example, plasma thrombopoietin levels appear to be regulated by the number of circulating platelets (see below). Therefore, the demonstration that a reduction of the oxygen-carrying capacity of the blood in the presence of a normal red cell count—for example, by carbon monoxide inhalation —stimulates erythropoietin levels to a similar extent as anemia was an important confirmation that anemia influences erythropoietin production mainly through changes of the oxygen-carrying capacity of the blood. Again, the efficacy of carbon monoxide to increase plasma erythropoietin levels is dependent on the hematocrit/hemoglobin concentration in a way that it is less effective during polycythemia and exaggerated during anemia.
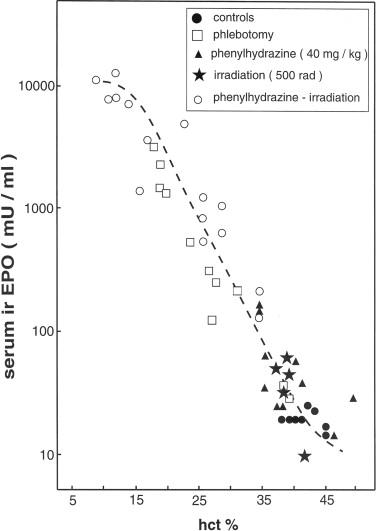
The time course of changes of plasma erythropoietin in response to an acute reduction of the oxygen-carrying capacity of the blood induced by blood loss or carbon monoxide inhalation is rather similar to that seen with acute arterial hypoxia. Plasma erythropoietin concentrations start to rise after about 60–90 minutes, reach a maximum value after about 20 hours, and then decline to a steady-state level. As with the influence of the oxygen tension on plasma erythropoietin levels, the effect of the oxygen-carrying capacity of blood on erythropoietin is not a linear one. The sensitivity of changes erythropoietin levels toward changes in hematocrit/hemoglobin concentration greatly decreases with increasing hematocrit/hemoglobin concentration, which becomes apparent when considering the plasma erythropoietin–hematocrit/hemoglobin relationship during the steady-state phase of chronic anemias ( Fig. 92.6 ). In humans and in laboratory animals, a fall of the hematocrit/hemoglobin concentration down to 70% of the normal value induces only a moderate increases erythropoietin levels. If the hemoglobin concentration decreases further, erythropoietin levels begin to increase exponentially. Nonetheless, significant elevations of plasma erythropoietin concentrations are measurable when hematocrit/hemoglobin are only slightly reduced from normal, such as following the donation of one unit of red blood cells. An increase in hemoglobin concentration above the normal value, such as during polycythemia rubra vera or hypertransfusion, significantly lowers serum erythropoietin levels, but does not fully suppress them.
Effect of hemoglobin oxygen affinity : Another parameter relevant for plasma erythropoietin levels that is linked to hemoglobin function is the oxygen affinity of the hemoglobin, which primarily determines oxygen release in the tissues. It has been observed that an increase of the hemoglobin oxygen affinity which impairs oxygen release to the tissues increases erythropoietin levels, while a decrease of the oxygen affinity attenuates the rise of erythropoietin levels in response to hypoxia.
All of the findings mentioned above converge to the oxygen availability to the tissues as common denominator governing circulating erythropoietin levels, because this value depends on the concentration of hemoglobin molecules available for oxygen transport, on the arterial oxygen tension determining the degree of oxygen saturation of hemoglobin, and on the oxygen affinity of the hemoglobin which finally determines the oxygen release from the hemoglobin. The fact that erythropoietin levels are only weakly affected by reductions of the arterial oxygen tension or hemoglobin concentration down to 50–70% of the normal values but then increase exponentially may be explained by the characteristic dissociation of oxygen from the heterotetrameric hemoglobin, which allows sufficient oxygen supply to the tissues down to a significant reduction of the oxygen content of the arterial blood. The observations that maneuvers interfering with oxygen delivery to the tissues and administration of transition metals such as cobalt and nickel increase plasma erythropoietin levels remained without sufficient explanation for a long time. In contrast to the effects of hypoxia and of carbon monoxide is the stimulatory effect of cobalt, which is not dependent on the hematocrit/hemoglobin concentration, suggesting that the effect of cobalt is not mediated by changes of the oxygen availability to the tissues. As outlined in detail below, a hypothesis has been developed that links the effects of cobalt and of hypoxia in a common model of oxygen sensing.
Additional physiological determinants involved in oxygen-dependent epo production? : Using a transgenic mouse model to modify keratinocytes, Johnson and co-workers suggested that the skin might be involved in systemic oxygen sensing by hypoxia induced vasodilation and redirection of the blood flow towards the skin, leading to renal hypoperfusion and increased Epo production. This putative mechanism is difficult to reconcile with the fact that other modalities leading to increased perfusion of the skin, such as heat exposure, must not alter renal Epo production and erythropoiesis. Subsequent to this study, it has been hypothesized that hair follicles in the skin itself could be a relevant source of circulating Epo. However, in studies with human volunteers no difference in the hypoxic increase in circulating Epo could be observed between subjects exposed to either systemic hypobaric hypoxia or inspiratory normobaric hypoxia of identical oxygen partial pressures, suggesting that extracutaneous hypoxia does not cause a physiologically relevant change in circulating Epo (C. Lundby, Zürich, Switzerland, personal communication). It has also been suggested that the brain releases an as yet unidentified factor upon hypoxia that triggers renal Epo production.
Astrocytes have been reported to represent another cell type responding to hypoxia with increased Epo synthesis in the human brain. While it was believed that Epo cannot cross the blood-brain-barrier, it was reported that astrocyte-derived Epo accounts for up to 50% of circulating Epo in mice following acute hypoxic exposure. This conclusion was derived from astrocyte-specific deletion of oxygen sensing which resulted in a 50% reduction of hypoxic Epo levels compared to wild-type mice. However, when human subjects were exposed to normobaric hypoxia, increases in blood Epo levels were similar in arteria brachialis and vena jugularis , suggesting that the brain does not substantially contribute to total circulating Epo (C. Lundby, Zürich, Switzerland, personal communication).
Linkage between erythropoietin secretion and rate of erythropoietin gene transcription : Hypoxia, anemia, and cobalt increase erythropoietin mRNA abundance, and changes of plasma erythropoietin levels correlate closely with the amount of erythropoietin mRNA in kidney and liver. Several lines of evidence suggest that erythropoietin producing cells do not develop stores of the hormone that can be rapidly released upon challenge. Thus, almost no erythropoietin can be extracted from the liver and kidney of normoxic animals. It takes a delay of at least 1 hour between an acute stimulation of erythropoietin and the first measurable rise of erythropoietin in the plasma. Several attempts to localize erythropoietin in the kidneys of normoxic animals failed to produce specific results. It is rather likely therefore that erythropoietin secretion into the bloodstream is directly triggered by the rate of de novo synthesis of the protein. Thus far, no evidence has been elaborated on how erythropoietin synthesis could be regulated on the ribosomal level; it is assumed now that erythropoietin synthesis is primarily determined by the number of mRNA copies available for translation. Steady-state mRNA levels in general result from a balance of gene transcription rate and the rate of mRNA degradation. Although there is some information that erythropoietin mRNA stability may be enhanced during hypoxia and an erythropoietin mRNA-binding protein has been identified, it is more or less accepted that cellular erythropoietin mRNA levels are mainly determined by the transcriptional activity of the erythropoietin gene. In summary, the erythropoietin secretion rate into the circulation is likely determined by the rate of erythropoietin gene transcription.
Survival of erythropoietin in the circulation : Considerable differences among species of the biologic half-life of erythropoietin in the circulation have been observed. In rats erythropoietin half-life is around two hours, in dogs, in sheep around nine hours, and around eight hours in rabbits. In humans the survival of erythropoietin is rather variable, and for human recombinant erythropoietin a number of studies have reported averaged half-lives at 4–11 hours. The mean half-life of endogenous erythropoietin in healthy humans was determined to be 5–6 hours. There is no information about a physiologically relevant regulation of erythropoietin survival in the circulation; in particular, hypoxia does not appear to affect the plasma clearance of erythropoietin. Even kidney function appears not to influence the half-life of erythropoietin in the plasma significantly, although a small proportion of the hormone appears each day in the urine. It is furthermore conceivable that the target cells of EPO contribute to its plasma half-life, since receptor-bound EPO is internalized and degraded. Supportive of this idea are observations that at a given hematocrit, plasma levels of EPO are lower in hyper-regenerative than in hyporegenerative anemias, and that for hyper-regenerative anemias such as sickle-cell anemia, half-life times for erythropoietin appears to be rather short.
Taken together, the survival rate of erythropoietin displays a rather high degree if intra- and inter-species variability, but appears not to be regulated in states of elevated or suppressed plasma erythropoietin concentrations.
Recruitment of erythropoietin-expressing kidney cells : Under normoxic conditions, renal (and also hepatic) erythropoietin mRNA abundance is low. After acute onset of hypoxia or anemia, erythropoietin mRNA levels start to increase within 30–60 minutes, and after termination of hypoxia, erythropoietin mRNA levels decline with a half-life time of around 1–2 hours. The rise of erythropoietin mRNA levels precedes and parallels the increase of erythropoietin plasma levels. The increase of whole-organ erythropoietin mRNA abundance could in principle result from an acceleration of erythropoietin gene transcription in a fixed number of cells, recruitment of erythropoietin expression cells, or a combination of both. For the kidney, good evidence has been elaborated for the latter possibility. Thus, the number of erythropoietin- expressing peritubular cells increases strikingly during anemia and hypoxia ( Fig. 92.5B ). In the kidneys of normoxic animals, only a few erythropoietin mRNA-expressing cells are found, preferentially in the deep cortex at the cortico-medullary junction. With increasing severity of hypoxic or anemic stress, an almost exponential increase occurs in the number or erythropoietin-expressing peritubular fibroblasts cells, which are recruited in direction to the superficial cortex. By that the number of erythropoietin-expressing cells increases several hundred-fold in response to hypoxia or anemia. Considering the ratio of whole-organ erythropoietin mRNA and number of erythropoietin-expressing cells reveals that there is a certain regulation of erythropoietin mRNA per cell, but that the increase of the number of cells that start to express erythropoietin is by far the more relevant mechanism to account for the rise of whole-kidney erythropoietin mRNA, and consequently, plasma erythropoietin levels ( Fig. 92.5B ). Therefore, regulation of erythropoietin production in the kidney appears mainly as an “on-off” switch of erythropoietin gene transcription by renal peritubular fibroblasts. In addition, cobalt administration increases the number of erythropoietin-expressing cells in the kidney. In contrast to the effects seen during hypoxia or anemia, is the recruitment induced by cobalt, which is zonally less organized.
In the liver it appears as if it is an up- and down-regulation of erythropoietin gene expression in certain hepatocytes, rather than a recruitment of erythropoietin-expressing cells, that accounts for the increase of hepatic erythropoietin mRNA in response to anemia. A gradual upregulation of erythropoietin mRNA in hepatocytes would also be supported by in vitro findings with primary cultures of hepatocytes or hepatoma cells ( Fig. 92.7 ).
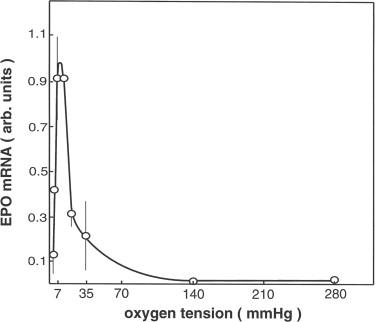
Evidence for local oxygen sensing in control of erythropoietin production : Oxygen-sensitive chemoreceptors are well known in the carotid body, and they are responsible for hemodynamic and respiratory adaptations to hypoxia. The regulation of erythropoietin appears to be independent of these systemic oxygen sensors; instead, it appears to be regulated by local oxygen sensing in liver and kidneys. Thus, ablation of the arterial chemoreceptors does not significantly alter erythropoietin production in response to hypoxia. Moreover, with respect to the renal production of erythropoietin, renal denervation has been found not to influence the expression of erythropoietin in response to hypoxia or anemia. On the other hand, there is consistent evidence that in isolated perfused kidneys taken from normoxic animals, erythropoietin gene expression and erythropoietin protein secretion can be induced by lowering the oxygen tension of the perfusate, with rather similar characteristics of the in vivo situation. Thus, it appears that the essential events by which systemic hypoxia or anemia stimulate peritubular erythropoietin gene transcription occur within the kidneys itself. This does not rule out the possibility that renal erythropoietin production may in addition be modulated by extrarenal factors (see below).
Because erythropoietin production is stimulated in a similar fashion by a fall of the arterial oxygen tension, by a reduction of the oxygen-carrying capacity of the blood, and by an impairment of oxygen delivery from the hemoglobin, it is likely that the common denominator for these effects is a reduction of the oxygen availability to the peritubular tissue. A measurable parameter that results from the oxygen availability in conjunction with the local oxygen consumption is the local oxygen tension. In addition to oxygen availability, oxygen consumption is of relevance for erythropoietin production. This may be derived from observations that hormonal modulation of the metabolic rate exerts a major role in erythropoietin production. Thus, even upon hypoxic exposure, erythropoietin production in hypophysectomized animals may be as low as in nephrectomized animals. Similarly, starvation also attenuates erythropoietin production. On the other hand, hormones stimulating energy turnover such as growth hormone, insulin-like growth factor I, thyroid hormone, and androgens enhance hypoxia-induced erythropoietin formation. Indeed, the renal cortical fibroblasts expressing erythropoietin are in close anatomical contact with proximal tubular cells, which have a high metabolic rate. Since circumstantial evidence suggests that the oxygen regulation of erythropoietin production in the kidneys is dependent on transport activity of the proximal tubule, it is conceivable that proximal tubule cells generate a sink of the oxygen tension around the neighbored fibroblasts.
Attempts to isolate erythropoietin-expressing fibroblasts and to study the regulation of erythropoietin gene regulation in these cells in vitro , however, have not been successful thus far. In mice bearing an erythropoietin SV-40T antigen construct transgene, renal fibroblasts expressing the T antigen could be isolated and cultured. In contrast to the in vivo situation, however, the cells did not increase their erythropoietin mRNA or T-antigen expression in response to hypoxia.
In principle, the pericellular oxygen tension can directly trigger erythropoietin gene expression. Thus, in cultures of hepatoma cells, hepatocytes, astrocytes, and liver Ito cells, a prominent effect of the pericellular oxygen tension on erythropoietin gene expression can be demonstrated, in a fashion rather similar to that expected from in vivo data ( Fig. 92.7 ).
At first glance, the location of an oxygen (tension) sensor in the kidney appears somewhat surprising, since the volume of oxygen transported to the kidney cortex is rather large compared to its oxygen consumption. Considering the comparatively low oxygen extraction rate of 8-10%, one might conclude that the kidney cortex generally is luxuriously supplied with oxygen. On the other hand, not only does the oxygen sensor governing erythropoietin gene transcription appear to reside within the kidney, but clinical experience also teaches that the kidney is an organ rather susceptible to hypoxic injury. The explanation for both these putative discrepancies probably resides in the unique architecture of intrarenal vessels. Since arterial and venous vessels run counter-currently over long distances in close association, it is assumed that a shunt diffusion of oxygen occurs between arterial and venous vessels, which lowers arterial oxygen pressure. While shunt diffusion of oxygen in postglomerular vessels is thought to be responsible for the high vulnerability of the thick ascending limb to ischemia, shunt diffusion in preglomerular vessels, in particular interlobular vessels, might explain the oxygen sensitivity of cortical structures relevant for erythropoietin production. Support for this concept comes from measurements of oxygen tension in the renal cortex well below the venous PO 2 of the kidney. Moreover, it has been demonstrated that preglomerular oxygen tensions at the kidney surface are much lower than those in the systemic circulation, this difference increasing greatly with rising oxygen tension and decreasing hemoglobin concentrations. It should be noted that assuming major oxygen shunting along interlobular vessels would predict an oxygen gradient from the corticomedullary junction to the subcapsular cortex, while the distribution of erythropoietin-expressing cells displays just the opposite direction, with the majority of signals in situ hybridizations being located in the inner part of the cortex. Thus, additional parameters are required to explain the particular distribution of erythropoietin-expressing cells. In particular, in the lower cortex the peritubular tissue frequently receives blood returning from the medulla or cortical medullary rays. Since these structures already extract a substantial amount of oxygen, oxygen delivery to the inner part of the cortex may be lower than in other cortical areas. Similarly, oxygen delivery to the midcortical peritubular cells may be somewhat reduced, because it also receives blood returning from the medullary rays, while in the superficial cortex postglomerular blood directly reaches the peritubular tissue. Another factor that has to be taken into account is that shunt diffusion is not limited to oxygen; it also (in the opposite direction) affects carbon dioxide, thereby trapping it in the outermost subcapsular zone. Since carbon dioxide attenuates erythropoietin production by a pH-related mechanism, an accumulation of carbon dioxide might at least partially counterbalance the effect of low oxygen tensions in the outer cortex. Consideration of all of these issues suggests that the main physiologic role of oxygen shunting occurring in the cortex could be an amplifying mechanism for the stimulation of erythropoietin production, which operates predominantly during states of anemia and less during arterial hypoxia. Assuming that the renocortical oxygen consumption is more or less unaltered during anemia, the oxygen tension in the primary capillary effluate leaving the parenchyma will be decreased in comparison with the nonanemic state. This decrease in venous oxygen tension will increase the driving force for oxygen shunting between interlobular arteries and veins, and in consequence further lowers the arterial oxygen tension of the blood entering the renocortical parenchyma.
In any case, the high oxygen sensitivity of the renocortical peritubular tissue is also substantiated by the observation that apart from erythropoietin expression, other events occur in the peritubular region of the renal cortex. Thus, within 1 week following the onset of anemia, a marked hypertrophy of peritubular fibroblasts in the cortical labyrinth of rat kidneys was found, with an increased activity of 5′-ectonucleotidase on the surface of these cells.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here