Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Therapeutic bloodletting is an ancient therapy that dates back to the Galenic conceptualization of illness caused by the imbalance of the four humors. The practice remained fashionable, albeit unproved, well into the 19th century. About the time that scientific skepticism began to temper the widespread use of therapeutic phlebotomy, a new technique for blood removal, apheresis, appeared in the research laboratory. The term apheresis , derived from a Greek verb meaning “to take away or withdraw,” was coined to describe removal of one component of blood with return of the remaining components to the donor. Like phlebotomy, apheresis was used first to treat patients but later became more important for collecting blood components for transfusion. In 2017, about 1.8 million units (14.6%) of red blood cells (RBCs), 2.2 million units (91.3%) of platelets, 350,000 units (10.8%) of plasma, and 4000 granulocyte doses were collected by apheresis in the United States. The Plasma Protein Therapeutics Association estimates that 40 million liters of source plasma are collected annually by plasmapheresis in approximately 30 million collections. Apheresis plasma is critical for preparing plasma-derived medicinal products such as intravenous immunoglobulin (IVIg) and albumin. Increasingly, apheresis techniques are also used to collect cells from the peripheral blood of healthy donors and patients for hematopoietic stem/progenitor cell (HSPC) transplantation and for manufacturing immune cell therapies such as chimeric antigen receptor (CAR) T cells. More than 50,000 HSPC and cellular therapy products are collected at hospital and blood center apheresis facilities annually. In clinical settings, apheresis may also be employed in a therapeutic context to treat disease by removing pathologic cells or plasma-bound substances. Therapeutic applications of apheresis will constitute the focus of this chapter.
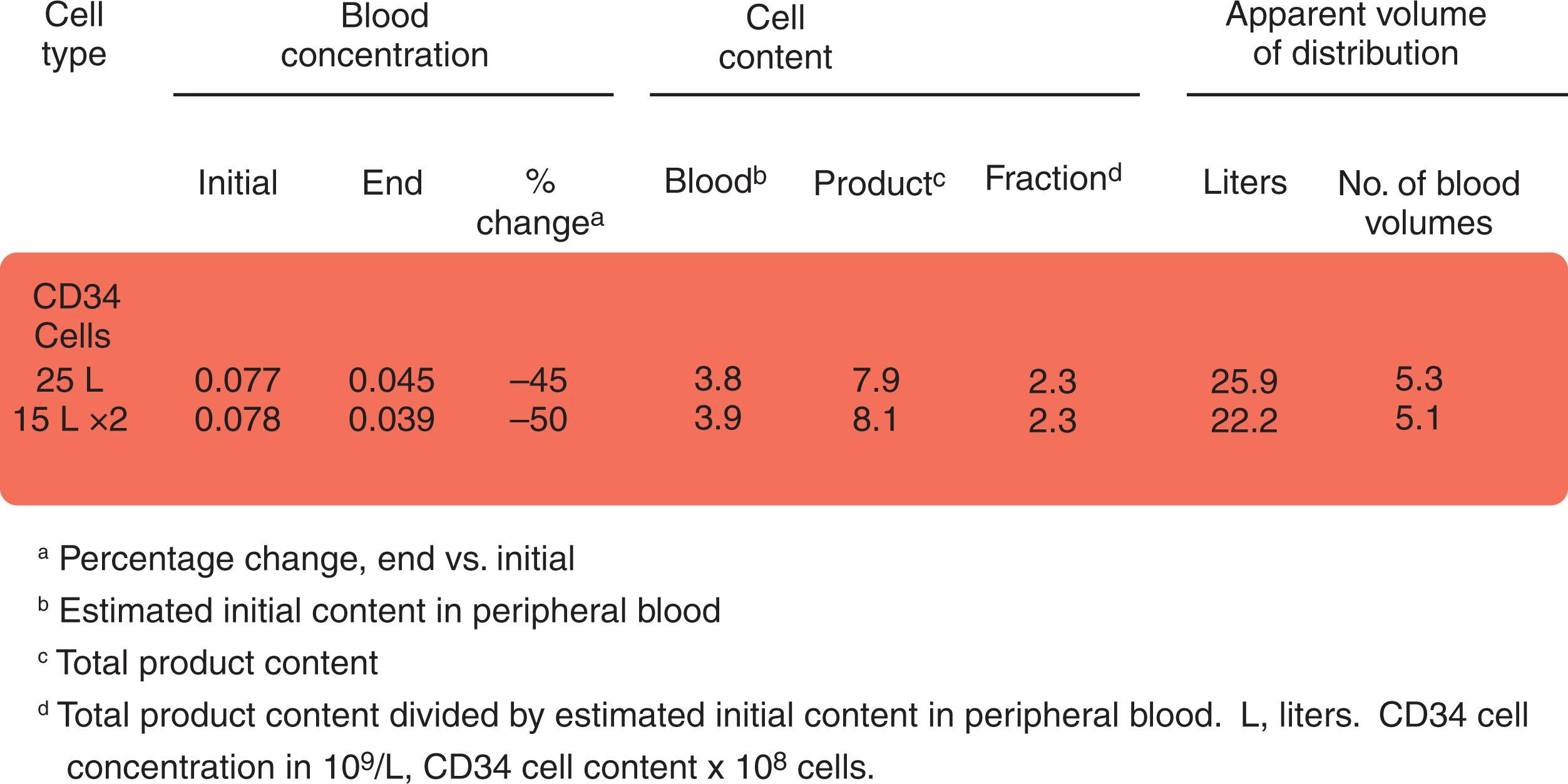
The principal objective of apheresis is efficient removal of some circulating blood component, either cells (cytapheresis) or some plasma solute (plasmapheresis). For most disorders, the treatment goal is to deplete the circulating cell or substance directly responsible for the disease process. Apheresis can also mobilize cells and plasma components from tissue depots. For example, lymphocytes may be mobilized from the spleen and lymph nodes of some patients with chronic lymphocytic leukemia (CLL), and low-density lipoproteins (LDLs) can be removed from tissue stores in patients with familial hypercholesterolemia. The apheresis procedure itself mobilizes CD34 + cells from extravascular depots during peripheral blood HSPC donation, resulting in collection of more than twice as many CD34 + cells than estimated based on preapheresis peripheral blood cell counts ( Fig. 116.1 ). Apheresis may have other, less obvious effects. Lymphocyte depletion may modify immune responsiveness in some disease states, possibly by disturbing the control mechanisms of cellular immune regulation. Plasmapheresis enhances splenic clearance of immune complexes in certain autoimmune disorders. When therapeutic effect is judged by clinical improvement rather than by efficiency of solute removal, apheresis is more often a helpful adjunct than a form of first-line therapy.
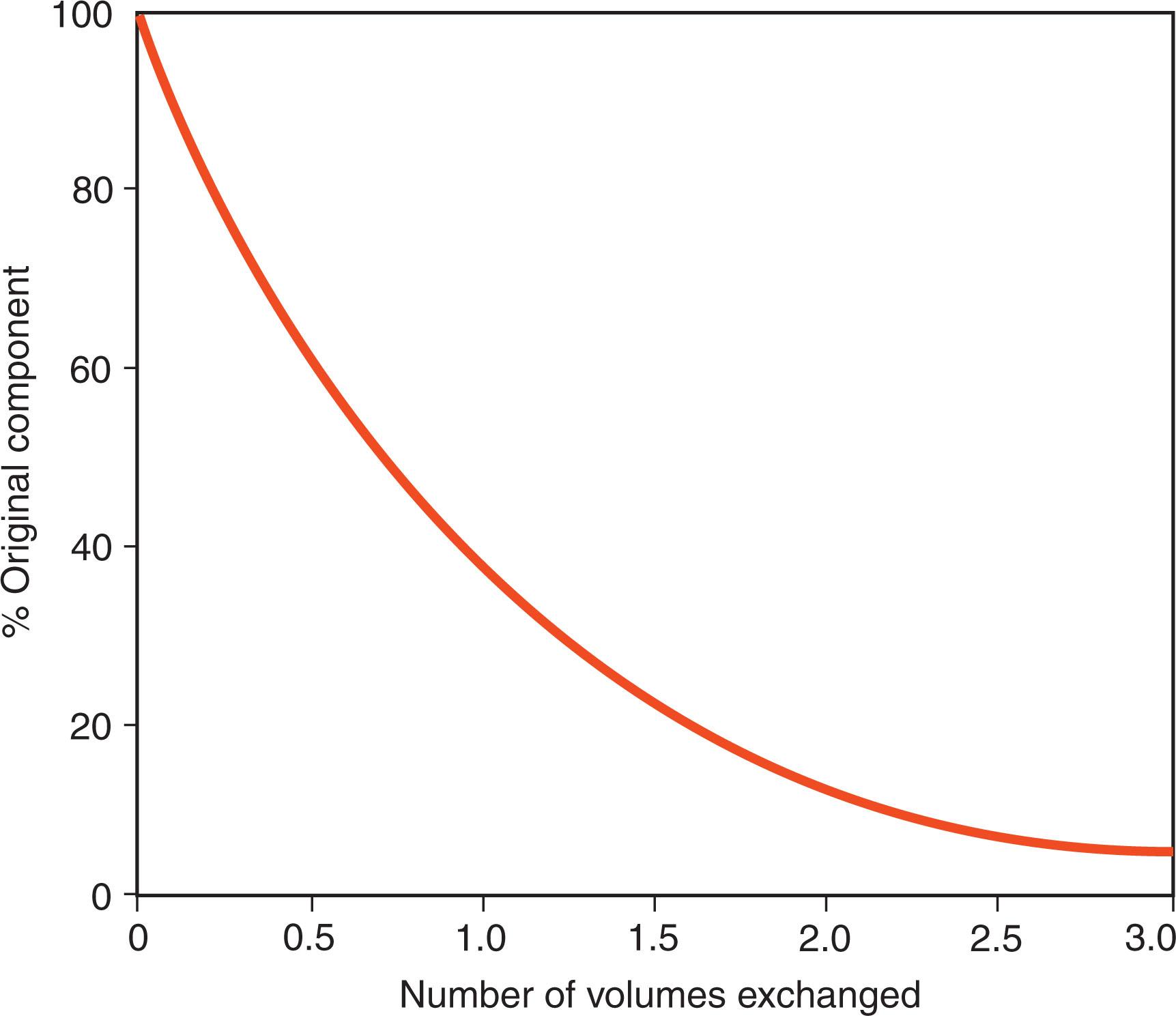
Several mathematical models formulated for different clinical conditions describe the kinetics of apheresis. Removal of most blood constituents follows a logarithmic curve ( Fig. 116.2 ). This model assumes that the substance removed is neither synthesized nor degraded substantially during the procedure, remains within the intravascular compartment, and mixes instantaneously and completely with any plasma replacement solution. When the goal of plasmapheresis is to supply a deficient substance, for example, the cleavase ADAM metallopeptidase with thrombospondin type 1 motif 13 (ADAMTS13) in the treatment of thrombotic thrombocytopenic purpura (TTP), replacement follows logarithmic kinetics similar to those developed for solute removal. Removal of 1.5 to 2.0 volumes will reduce an intravascular substance by approximately 80% (see Fig. 116.2 ); processing larger volumes results in little additional gain. Specific cell removal with centrifugal automated cell separators depend on the number of cells available, the volume of blood processed, the efficiency of the particular instrument, and the separation characteristics of the different cells. Most commercially available instruments remove platelets and lymphocytes extremely efficiently. Granulocytes and other mononuclear cells, including HSPCs from peripheral blood, cannot be cleanly separated from other cells by standard centrifugal apheresis equipment ( Fig. 116.3 ). Optimal harvesting of these cells requires special techniques such as stimulating the donor with corticosteroids, cytokines or chemokine receptor antagonists, or adding sedimenting agents to enhance cell separation.
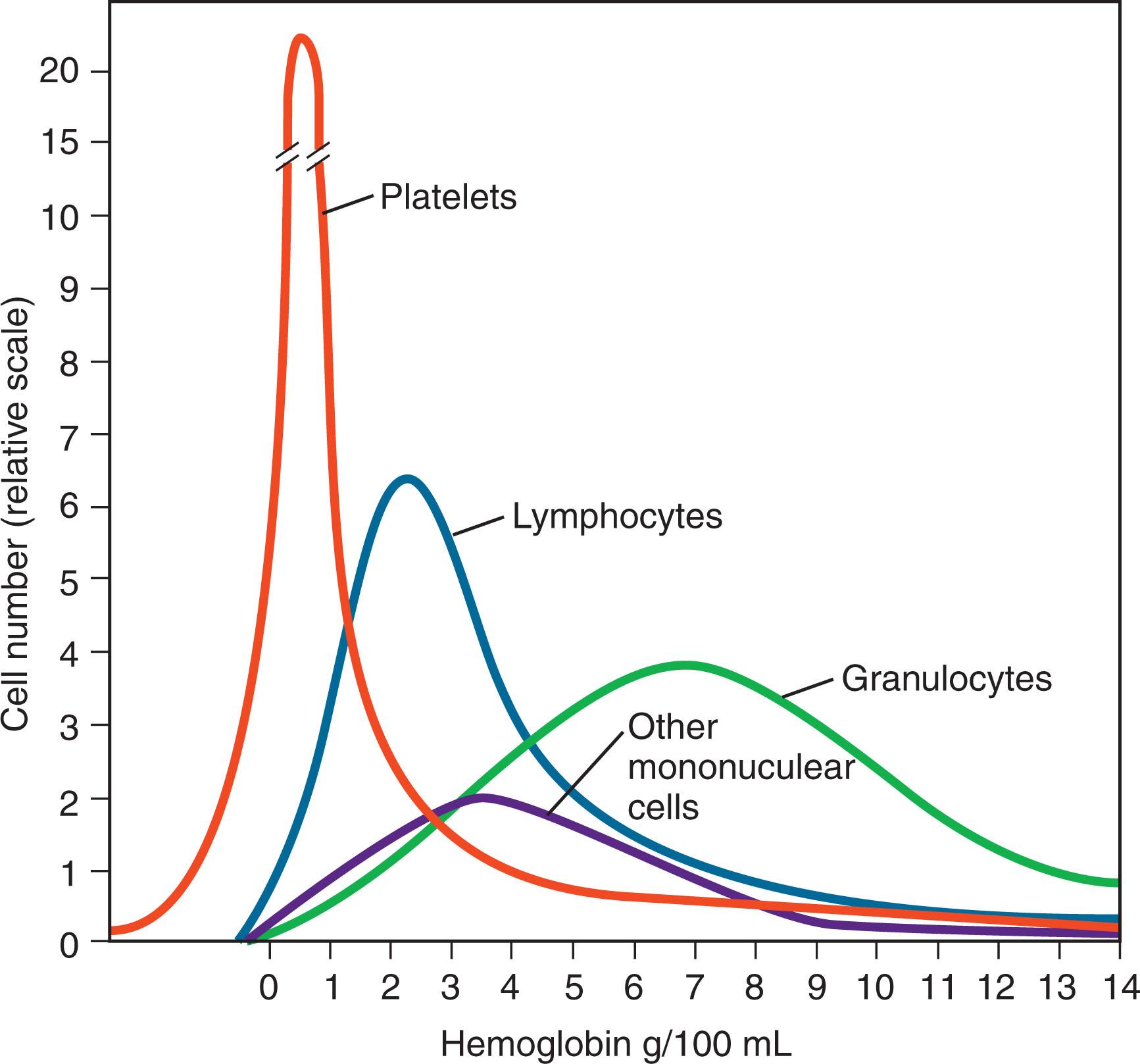
Whereas this model accurately estimates removal of cells and large proteins such as fibrinogen and immunoglobulin (Ig) M, removal of smaller solutes such as IgG and albumin-bound drugs are less efficient. Transfer of these moieties from the extravascular to the intravascular compartment depends both on diffusion along a concentration gradient and on active transport. The rate of clearance can be calculated using diffusion coefficients, sieving coefficients, and lymphatic flow rate, although in practice this degree of accuracy is rarely necessary.
Over 100 years ago, animal experiments formed the theoretical and practical basis for all modern extracorporeal blood purification methods. The plasmapheresis technique that originated in the animal laboratory required manual resuspension of RBCs and posed a substantial risk of microbial contamination of the components being reinfused. With the introduction of sterile, disposable, interconnected plastic blood bags, plasmapheresis became relatively safe and easy. However, manual apheresis proved too inefficient and labor intensive for collecting large component volumes, and raised concerns that the separated units of RBCs might be reinfused accidentally into the wrong donor or patient. The introduction of automated online blood cell separators solved these problems. Automated apheresis instruments use microprocessor technology to draw and anticoagulate blood, separate components either by centrifugation or by filtration, collect the desired component, recombine the remaining components for return to the patient or donor, and document procedure data. The equipment contains disposable plastic software in the blood path and uses anticoagulants containing citrate or combinations of citrate and heparin that do not result in clinical anticoagulation of the patient or donor. Most instruments function well at blood flow rates of 30 to 80 mL/min, and can operate from peripheral venous access or from a variety of multilumen central venous catheters. Newer therapeutic apheresis devices are smaller and more automated, allowing for implementation of more safety functions and improved portability. In large-volume leukapheresis (LVL) procedures, prophylactic intravenous calcium supplementation minimizes the risk of hypocalcemia due to the citrate anticoagulant used.
Because the ideal method for treating disorders mediated by abnormal plasma components is to remove the offending substance selectively, a variety of online filtration and column adsorption techniques have been introduced or proposed. Ligands bound to a column matrix may be relatively nonspecific chemical sorbents, such as charcoal or heparin, or specific ligands, such as monoclonal antibodies and recombinant protein antigens. Two such columns are commercially available: one using staphylococcal protein A, and the other using negatively charged dextran sulfate cellulose beads. Staphylococcal protein A has high affinity for the Fc portion of IgG1, IgG2, and IgG4 and for immune complexes containing these IgG subtypes. The dextran sulfate cellulose columns selectively remove LDL, very-low-density lipoprotein, and lipoprotein, and have proved effective in managing patients with homozygous hypercholesterolemia who have not responded to diet and cholesterol-lowering drug therapy ( Fig. 116.4 ). A similar technique, heparin-induced extracorporeal lipoprotein precipitation, uses low pH and negatively charged heparin to precipitate lipoproteins and remove the precipitate by filtration online (H.E.L.P. Plasmat Futura, Braun Medical). Apheresis technology has also been adapted for extracorporeal phototherapy of patient leukocytes (photopheresis) to treat cutaneous T-cell lymphoma, and to modulate the pathologic immune response in graft-versus-host disease (GVHD), solid organ transplant.
Apheresis services are generally overseen by specialists in Hematology, Nephrology, or Transfusion Medicine; qualified nurses or technicians perform the apheresis procedures. When requesting a course of therapeutic apheresis, the team should specify the indication and urgency of the request as well as the therapeutic endpoint. Defining specific clinical or laboratory endpoints for success, a priori, is important to avoid continuing a fruitless course of apheresis with inherent risks but no clinical benefit. Successful therapeutic apheresis requires reliable vascular access, using either two large, durable peripheral veins or a central dual lumen catheter that is rigid enough to withstand significant flow pressures. The fluid volume removed by therapeutic apheresis must be replaced to prevent marked volume depletion. Some patients, such as small children or pregnant women, may require special technical considerations to appropriately manage volume shifts or minimize intraprocedural anemia. The apheresis schedule should be determined by the patient's condition, the pathologic substance targeted for removal, and the desired clinical and/or laboratory endpoint.
Common clinical indications for therapeutic plasma exchange (TPE), frequently referred to as plasmapheresis, are outlined in Table 116.1 . Most procedures are performed for treatment of immunologic and hematologic disorders. A course of plasmapheresis generally consists of five to seven exchanges of 1 to 1.5 plasma volumes each, either daily or with an interval of 1 to 2 days between procedures; the course of therapy varies depending on the specific disease indication and rate and duration of the response. Several expert committees have published practice guidelines for using plasmapheresis in a wide variety of disease states. Some of the least controversial indications for plasmapheresis are supported by small series of uncontrolled cases that rely on some objective clinical or laboratory measurement of patient improvement. In recent years, effective biologic and pharmacologic agents such as IVIg have rendered TPE a backup therapy for some indications.
| Procedure | Indication (ASFA Category) |
|---|---|
| Red blood cells (RBC)exchange |
|
| Erythrocytapheresis | Hemochromatosis, polycythemia vera |
| Leukapheresis | Symptomatic hyperleukocytosis (II) |
| Adsorptive cytapheresis | Behçet’s disease (II) |
| Extracorporeal Photopheresis |
|
| Cell Therapy Manufacture |
|
| Thrombocytapheresis | Symptomatic thrombocytosis |
The success of therapeutic apheresis procedures seldom depends on the composition of the replacement solution; the major exception is TTP for which plasma is indicated to replace the depleted ADAMTS13 protein. With therapeutic plasmapheresis for most other disorders, the primary function of the replacement solution is to maintain intravascular volume. Additional requirements include restoration of important plasma proteins, maintenance of colloid osmotic pressure, maintenance of electrolyte balance, and preservation of trace elements lost during a prolonged course of plasmapheresis procedures. For most conditions, it is acceptable to perform 1 to 1.5 plasma volume exchanges per procedure. A single plasma volume exchange in an average-sized adult uses approximately 3 L of replacement fluid. In moderately well-nourished patients, homeostatic mechanisms normally obviate the need for precise plasma replacement, and 5% albumin in normal saline or combinations of albumin and crystalloid are usually sufficient. Patients with clinical conditions such as hypotension, hypoalbuminemia, or preexisting coagulopathies should receive solutions prepared specifically to meet their individual requirements. Problems of decreased availability and high cost of albumin have led some centers to develop protocols for alternatives to plasma-derived volume expanders, such as hydroxyethyl starch (HES), for full- or partial-volume replacement with plasma exchange. One center has used a combination of 3% HES and 5% albumin mixture successfully, but patients did experience mild adverse events more frequently than did historical control subjects. Although such solutions are generally well tolerated, extensive replacement with HES in patients undergoing longer courses of plasmapheresis, especially those with impaired renal function, can result in diffuse tissue accumulation of the larger starch molecules (acquired lysosomal storage).
Two of the most common indications for plasmapheresis are treatment of TTP and treatment of clinical syndromes associated with paraproteinemias. Plasma exchange with fresh-frozen plasma (FFP) replacement has been estimated to improve survival rates of patients with TTP from 10% to more than 75%. Whereas plasma infusion is often initiated immediately, TPE is the superior treatment and should be started as soon as it can be arranged. Comprehensive reviews of the clinical and laboratory evaluation and treatment of patients with suspected TTP, including management with plasma exchange therapy, have been published. Treatment usually involves daily single-volume plasma exchange with both frequency and duration of treatment guided by clinical response and continued until the platelet count is above 150,000/μL and lactate dehydrogenase is near normal for 2 to 3 consecutive days. The persistence of schistocytes on the peripheral blood smear does not preclude weaning or discontinuation of treatment. Typically, patients should respond within 2 or 3 days of beginning treatment. In desperately ill and deteriorating patients, escalating the intensity of plasma exchange to twice daily may be necessary. Despite initial reports of improved response rates in certain patients, recent experience suggests that the use of cryoprecipitate-poor plasma may not be more effective than the use of standard FFP as a specific replacement fluid for plasma exchange in patients with TTP. The effectiveness of plasma exchange in this setting may derive from removal of antibody to or replacement of the von Willebrand factor–cleaving zinc metalloprotease, ADAMTS13. However, patients with clinical features of TTP and only moderate ADAMTS13 deficiency or even normal activity may respond to plasma exchange. Refractory TTP may become less common in the era of caplacizumab, a recent front-line therapeutic antibody directed at Von Willebrand Factor (anti-VWF), and novel agents such as recombinant ADAMTS13 which is being evaluated in clinical trials.
TPE is generally not effective in the treatment of other thrombotic microangiopathies (TMA). Plasma exchange for hematopoietic progenitor cell transplant recipients exhibiting clinical features of TMA, now generally referred to as transplant-associated thrombotic microangiopathy (TA-TMA), has proved ineffective and potentially harmful. This syndrome likely differs in pathogenesis from classic TTP in many aspects, including the absence of severe ADAMTS13 deficiency, the spectrum of clinical symptoms, and the lack of evidence of systemic microthrombus formation. Atypical hemolytic uremic syndrome (aHUS), a rare form of thrombotic microangiopathy with high mortality, may be difficult to distinguish clinically from TTP in adults. aHUS results from uncontrolled complement activation, does not respond to plasma therapies, and is instead treated with eculizamab to block the terminal complement complex. Other causes of TMA, such as HUS due to Shiga toxin–producing Escherichia coli infection (STEC-HUS) or drug-induced TMA have not been shown to respond to plasma exchange.
Small, uncontrolled studies and extensive clinical experience support the use of plasmapheresis as an adjunctive therapy for patients with paraproteinemia and hyperviscosity syndrome, with some paraproteinemias in the absence of hyperviscosity. Waldenström macroglobulinemia manifests as a lymphoplasmacytic lymphoma with a monoclonal IgM protein in the plasma. Because IgM is a large molecule and resides predominantly in the intravascular space, as little as one apheresis procedure can result in dramatic improvement in symptoms. Recurrence of symptoms and rising plasma viscosity will determine the need and frequency of repeated exchanges. Definitive chemotherapy should be initiated concurrently. Comprehensive reviews describing the rationale and treatment schedules for plasmapheresis in patients with a variety of paraproteinemias, including cryoglobulinemia and Waldenström macroglobulinemia have been published.
In some immune disorders, such as immune thrombocytopenic purpura (ITP) and immune inhibitors to coagulation proteins, plasma exchange may be helpful during a catastrophic event, but in general, benefit of nonselective plasma exchange therapy is not established.
Plasmapheresis is effective first- or second-line therapy in selected patients with certain neurologic disorders. Controlled clinical trials of plasmapheresis have demonstrated efficacy in at least two of the polyradiculoneuropathies. In Guillain-Barré syndrome, plasmapheresis should be considered when patients are unable to walk independently or require mechanical ventilation. However, IVIg alone may be equally effective and is more readily available. Periodic plasmapheresis may be necessary in patients with a chronic inflammatory demyelinating neuropathy. Because the long-term prognosis varies, plasmapheresis may be used in conjunction with steroids and IVIg. Rapid deterioration may occur upon discontinuation. Myasthenia gravis (MG) is one of the most common indications for TPE in many hospital settings, particularly in the context of myasthenic crises or refractory MG. Other neurologic disorders characterized by circulating autoantibodies, such as neuromyelitis optica, N-methyl D-aspartate (NMDA)-encephalitis, and Eaton-Lambert syndrome, have also been treated successfully with TPE.
Multiple sclerosis (MS) is a relapsing and progressive disorder with demyelination of the central nervous system white matter. Patients who present with acute fulminant demyelination may benefit from early plasma exchange, particularly when they fail to respond to high-dose corticosteroids. The majority of patients have a relapsing-remitting form of the disease, and plasmapheresis may be of benefit. Unfortunately, for chronic progressive forms of MS, plasma exchange has consistently been shown to be ineffective. Natalizumab (NTZ), an effective treatment for relapsing-remitting MS, may rarely be complicated by progressive multifocal leukoencephalopathy (PML); plasmapheresis has also been attempted to facilitate clearance of NTZ in this context.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here