Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
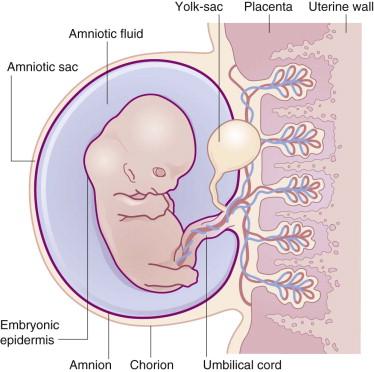
The skin simultaneously contacts a changing environment and provides closure for the body. It is a cellular and molecular interface, which plays a critical functional role in neurobehavior and perception. The skin is embryologically continuous with the nervous system via involution and with the amnion via simple lateral extension. Thus, with regard to the fetoplacental unit, the skin is an ‘internal’ organ marking the boundary between the developing nervous system and the fetal membranes surrounding the liquor amnii ( Fig. 1.1 ).
Few organs carry such capacity to convey and evoke emotion. The experiments of Hooker and Humphrey, for example, provide unequivocal video evidence that localized tactile stimulation of the skin of the human embryo as early as 8–9 weeks' gestation will evoke a reflex response. Thus, even early in development, the skin cannot be considered apart from the developing central nervous system and other fetal organs, nor, as will be demonstrated in this chapter, from the changing dynamics of its aqueous environment and maternal enclosure.
Fetal skin development occurs in a unique physiological world. The normal intrauterine environment is profoundly hypoxic, a condition aptly described by the eminent fetal physiologist, Joseph Barcroft, as ‘Mt Everest in utero’. Fetal wounds heal with little or no scarring. Touch, the first sense to develop in all vertebrates, occurs in a milieu where stimuli are muted and receptor pathways may be immature. The electrical impedance of the fetal skin is inconstant and increases markedly in mid-gestation. The fetus develops in an environment of relative immune privilege and critical protective functions of the skin, which are unnecessary prenatally, must be operative immediately at birth, e.g., protection from environmental trauma, infection, cold, and xeric stress.
Comparison of human fetal skin development to other well-studied animal models highlights important and peculiar differences. Humans, for example, have a relatively long gestation ( Table 1.1 ![]() ). Human infants have a prolonged postnatal period of vulnerability when maternal bonding and skin-to-skin contact figure prominently. According to Brazelton, the human neonate, in contrast to other species, is relatively precocious in sensory capabilities and relatively helpless in motor skills. This developmental disparity places a premium on understanding the intrauterine maturation of the skin as a sensory organ and the impact of cutaneous function on the developing nervous system. Dermatologists and skin scientists will note that humans are distinguished from other primates, not merely by opposable thumbs and a large, versatile brain but most obviously by a body surface, which is strikingly furless, vulnerable, and characterized by an expanse of well-developed interfollicular epidermis, earning humans the designation, the ‘Naked Ape’.
). Human infants have a prolonged postnatal period of vulnerability when maternal bonding and skin-to-skin contact figure prominently. According to Brazelton, the human neonate, in contrast to other species, is relatively precocious in sensory capabilities and relatively helpless in motor skills. This developmental disparity places a premium on understanding the intrauterine maturation of the skin as a sensory organ and the impact of cutaneous function on the developing nervous system. Dermatologists and skin scientists will note that humans are distinguished from other primates, not merely by opposable thumbs and a large, versatile brain but most obviously by a body surface, which is strikingly furless, vulnerable, and characterized by an expanse of well-developed interfollicular epidermis, earning humans the designation, the ‘Naked Ape’.
| Species | Gestation in days (approx.) |
|---|---|
| Laboratory mouse | 20.5 |
| Domestic rat | 21.5 |
| Domestic rabbit | 32 |
| Dog | 57–63 |
| Domestic cat | 63 |
| Guinea pig | 68 |
| Sheep | 147 |
| Monkey (rhesus) | 165 |
| Man | 265 |
In contrast to term gestation, the border of viability is the gestational age when the prematurely delivered fetus can survive in the extrauterine environment. In humans, this border, dependent in part upon medical intervention, exhibits racial and gender differences and varies from country to country, but is typically placed at 23–25 weeks' gestation. This is parenthetically the time of formation of the epidermal barrier, i.e., that skin structure critical for postnatal transition and survival.
Although typically outside the purview of traditional dermatology, the infant at birth benefits from desirable surface characteristics, including skin suppleness, softness, and smoothness as well as pheromonal influences, which positively influence maternal bonding and caregiver support. This positive perception of infant skin emphasizes the skin–brain connection and cannot be dismissed from dermatological inquiries in the newborn period. The quintessential maternal–infant bonding experience in mammals , i.e., breast-feeding, involves intimate skin-to-skin contact between mother and infant and the production of milk by an ectodermal, skin-based glandular derivative.
In addition to normal physiological development, pathophysiological events may arise in utero with subsequent need for medical intervention. Innate immune mechanisms to combat chorioamnionitis, for example, are important in the last trimester, to forestall systemic inflammatory responses in the fetus which carry long-term neurological sequelae. Birth trauma from intrauterine events such as amniotic bands or iatrogenic incidents may arise along with a panoply of gene defects, leading to congenital skin disorders. Later chapters detail specific dermatological conditions, which have their roots in utero. New tools of molecular investigation and the possibility of intrauterine therapies are exciting new fields of research. Finally, recognition of the body surface as a critical interface for receiving and delivering care transcends specific diseases and includes a plethora of important functions such as skin adhesion, monitoring, topical wound care, bathing, cleansing, emolliency, and microbiome support. All of these skin-based functions have their beginning in the transition of the fetus to the infant at birth.
Important morphologic events in intrauterine skin development are illustrated in Table 1.2 . In Table 1.2 , estimated gestational age (EGA) refers to the system used in basic embryology texts and by researchers to refer to the age of the fetus. In this system, fertilization occurs on day 1. However, the dating system used by obstetricians and other clinicians as a convenient method for staging pregnancy defines day 1 as the first day of the last menstrual period (LMP) and is synonymous with menstrual age. In this dating system, fertilization occurs on approximately day 14. Thus, a woman who is 14 weeks' pregnant (LMP) is carrying a 12-week-old fetus (EGA).
| Structure or event | Estimated gestational age (weeks) |
|---|---|
| Epidermal stratification and expression of K5, K14 and K1, K10 | 6 |
| Presence of melanocytes and Langerhans' cells in the epidermis | 8 |
| Formation of complete hemidesmosomes, anchoring filaments, and anchoring fibrils | 8–10 |
| Formation of the nail primordium | 10 |
| Initiation of hair follicles | 12 |
| Initiation of eccrine sweat glands on the palms and soles | 10–12 |
| Delineation of papillary and reticular dermis | 11–12 |
| Formation of adipose tissue in hypodermis | 15 |
| Follicular keratinization | 15 |
| Interfollicular keratinization | 22–24 |
| Formation of eccrine sweat glands on the body | 24–26 |
From a functional point of view, fetal skin development can be divided into three temporally overlapping stages – organogenesis , histogenesis , and maturation – that correspond roughly to the embryonic period (0–60-plus days); the early fetal period (60 days to 5 months); and the late fetal period (5–9 months) of development. The first stage, organogenesis , involves the specification of ectoderm lateral to the neural plate to become epidermis and the allocation of subsets of mesenchymal and neural crest cells to become dermis. During this stage, embryonic ectoderm and mesoderm become physically apposed, and they initiate the signaling cross-talk necessary for basement membrane and subsequent skin appendage (hair, nail, and sweat gland) formation.
The second stage, histogenesis , is characterized by dramatic morphologic changes in the presumptive skin, including epidermal stratification, epidermal appendage involution and differentiation, mesenchymal subdivision of the dermis and hypodermis, and vascular neogenesis. The third stage, maturation , entails the functional evolution of these skin components, so that they provide adequate thermoregulatory capacity, surface tensile strength, and barrier function for postnatal survival in the harsh, arid, nonsterile extrauterine environment. The remainder of this chapter highlights selected events of structural development of the skin in utero along with important physiological and clinical correlates.
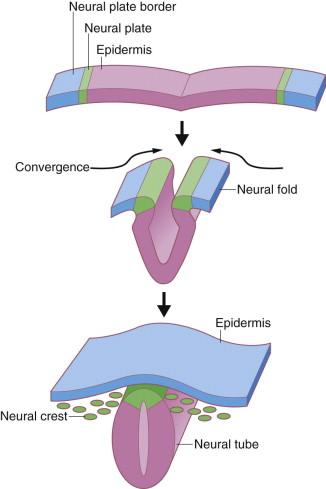
During the third week after fertilization, the human embryo undergoes gastrulation, a complex process of involution and cell redistribution that generates the three primary embryonic germ layers: endoderm, mesoderm, and ectoderm. Shortly after gastrulation, the ectoderm is further subdivided into neuroectoderm, a medial strip parallel to the long axis of the developing embryo, and presumptive epidermis on either side of this strip. Neurulation results in infolding of the embryonic ectoderm to become the neural tube and subsequent brain and spinal cord ( Fig. 1.2 ). The extraembryonic ectoderm (lateral to the epidermis) becomes the amnion lining the amniotic sac. The early presumptive epidermis is a loosely associated single cell layer. By 6 weeks' EGA (8 weeks' LMP), the surface ectoderm covering most regions of the body already consists of basal cells and more superficial periderm cells ( Fig. 1.3 ). The periderm layer is a transient embryonic layer that does not participate in the production of definitive epidermal progenitors. The presumptive epidermis at these early stages is not considered a true stratified epithelium.
The basal cells of the embryonic epidermis display morphologic and biochemical features similar – but not identical – to basal cells of later developmental stages. Embryonic basal cells are slightly more columnar than later fetal basal cells and lack morphologically distinct hemidesmosomes. Matrix adhesion molecules critical for histogenesis and signal transduction, such as E- and P-cadherins and integrins β-1 and β-4, exhibit spatially and temporally coordinated expression in the developing epidermis. The keratin pair K8/K18, typically found in simple epithelial cells, is the first pair expressed in embryogenesis and may represent the oldest phylogenetic keratins. Keratins involved in higher order tonofilament formation such as K5/K14 can also be identified.
Periderm cells of the embryonic epidermis are larger and flatter than the underlying basal cells. As such, periderm cells have been termed a ‘pavement epithelium’. Apical surfaces in contact with the amniotic fluid are studded with microvilli. Their lateral surfaces in contact with adjacent peridermal cells are sealed with tight junctions, possibly precluding passive – but not active – diffusion of fluids across this outer layer of the embryo. Periderm cells, like the embryonic basal cells, express the stratified epithelial keratins K5 and K14, but also express simple epithelial keratins K8, K18, and K19. Towards the end of the second trimester these superficial cells are eventually sloughed.
By the end of 8 weeks' gestation (10 weeks' LMP), the basic components of most organ systems have been laid down and hematopoietic production has shifted to the bone marrow. This marks the classic division between embryonic and fetal development, and it corresponds to initial epidermal stratification and the formation of the third ‘intermediate’ layer between the two pre-existing cell layers ( Fig. 1.3 ). Cells in the intermediate layer of the early fetal epidermis express the K1/10 skin differentiation-type keratin markers, as well as the desmosomal protein desmoglein 3, which is also known as the pemphigus vulgaris antigen. Moreover, intermediate filaments and desmosomal junctions are more abundant in this layer than in the basal or periderm layers. In contrast to the spinous cells of the mature nonwounded epidermis, cells within the intermediate layer remain highly proliferative. Over the next several weeks, more layers are gradually added to this intermediate zone of the developing epidermis, such that by 22–24 weeks' EGA, the epidermis contains four to five layers in addition to terminal differentiation of the periderm with formation of a cross-linked cornified envelope ( Fig. 1.4 ).
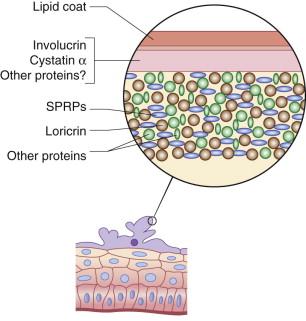
After the onset of stratification, the basal layer also displays characteristic morphologic and biochemical changes. Basal cells become more cuboidal and begin to synthesize other keratin peptides, including K6, K8, K19, and the K6/K16 hyperproliferative pair. This latter keratin pair is not normally expressed in mature interfollicular epidermis but is upregulated in response to wounding and hyperproliferative conditions. During early fetal development, the basal cell layer also begins to express the hemidesmosomal proteins BPA1 and BPA2, and to secrete collagen types V and VII, the latter being the major component of the anchoring fibrils of the dermis. DNA-labeling studies indicate that by 80–90 days' EGA, a distinct subset of slow-cycling cells exists within the basal cell population, suggesting that an epidermal stem cell population has already been set aside at these early stages.
Maturation of the epidermis during late fetal development is characterized by the generation of granular and stratum corneum (SC) layers, the formation of a water-impermeable barrier, and the sloughing of the periderm. Keratinization, the terminal differentiation seen in the stratum granulosum (SG) and SC, is initiated first in the skin appendages between 11 and 15 weeks' EGA, and extends to the interfollicular epidermis from about 22–24 weeks' EGA. During the third trimester, the cornified cell layers increase in number, aiding in the formation of a barrier.
The prenatal epidermal water permeability barrier was previously thought to be derived almost entirely from lipid secreted from cells of the outer SG and processed to highly hydrophobic species in the SC interstices, controlled not only by the intrinsic program of epidermal barrier development but also by prenatal exposure to fetal or maternal hormones, nutrient gradients, or by air exposure at birth. In postnatal humans and rodents, this epidermal permeability barrier is formed by polar lipids secreted from the SG that are then processed into impermeant lamellar bilayers. In contrast, since prior freeze-fracture electron microscopy studies showed only discontinuous tight junction (TJ) strands in adult mouse epidermis, it was widely thought that in contrast to amphibian skin or other ‘tight’ mammalian epithelium (such as kidney), TJ played only a minor role in the epidermal water permeability barrier.
Although congenital human skin diseases caused by mutations in TJ are rare, common diseases such as atopic dermatitis have been linked with acquired TJ dysfunction. Further, studies show that claudin-1 deficient mice suffer barrier defects leading to death soon after birth. Involucrin-Cldn6 (Inv-Cldn6) transgenic mice also display skin barrier defects, the severity of which is dependent upon the level of Cldn6 over-expression. These results suggest that TJ also plays an important role in forming the epidermal permeability barrier during the prenatal period, or in regulating the subsequent development of the lipid barrier.
Gross defects in early epidermal specification and organogenesis are rarely observed in the neonate, probably because they are incompatible with fetal survival. Using mice as an animal model system, researchers demonstrated that obliteration of the p63 gene precludes the formation of most multilayered epithelia in the body, leading to perinatal lethality due to loss of skin barrier function. Humans who carry mutations in this gene still retain some functionality and therefore display less severe alterations in their epidermis and appendages (see below).
In contrast, congenital defects in epidermal maturation are not uncommon, as they do not usually impinge on in utero survival. Lamellar ichthyosis (see Chapter 19 ) is usually inherited in an autosomal recessive manner and in 30% of patients is caused by mutations in the gene encoding epidermal transglutaminase, the enzyme that cross-links submembranous proteins to form the insoluble cornified envelope of the SC. In its absence, large, dark polygonal scales form over the entire body, and at birth, the infant may be transiently wrapped in a waxy, collodion-like membrane. A similar clinical presentation can be seen in patients homozygous for mutations in the ABCA12 gene, which encodes an ATP-binding cassette thought to be important for lipid trafficking across keratinocyte membranes. Infants with the more severe ‘harlequin ichthyosis’ (see Chapter 19 ) are born encased in armor-like, thickened, adherent SC which cracks upon exposure to air. This extreme variant also appears to be due to mutations in the ABCA12 gene.
In contrast to the permanent manifestations of genetic defects, the inadequate epidermal keratinization and maturation of the premature epidermis are transient. Immaturity of the SC, especially in infants born before 28 weeks' EGA (30 weeks' LMP), places these neonates at increased risk for dehydration, excessive penetration of topical drugs or other chemicals, and infection from organisms newly colonizing the skin (see Chapters 4 and 5 ). In general, even full-term newborns display a somewhat reduced barrier function, and continued maturation occurs over the first few weeks of life, such that by 3 weeks of age, the newborn's SC is structurally and functionally equivalent to that of the adult; maturation is accelerated in the premature infant, although the duration may be longer in extremely premature infants.
Two major immigrant cells – melanocytes and Langerhans' cells – populate the epidermis during early embryonic development. Melanocytes are derived from a subset of neuroectoderm cells, the neural crest, which forms along the dorsal neural tube and gives rise to a variety of cell types, including many tissues of the face and peripheral autonomic neurons. Neural crest cells destined to become melanocytes migrate away from the neural tube within the mesenchyme subjacent to the presumptive epidermis. They migrate as semicoherent clones laterally and then ventrally around the trunk to the thoraco-abdominal midline, anteriorly over the scalp and face, and distally along the extremities. Postnatally, the embryonic paths taken by these partially coherent clones can be readily visualized in patients with banded pigmentary dyscrasias following Blaschko's lines, such as the disorders classified as hypomelanosis of Ito, and linear and whorled hypermelanosis (see Chapters 23 and 24 ).
Melanocytes are first detected within the epidermis of the human embryo at approximately 50 days' EGA, recognized by their dendritic morphology and their specific immunoreactivity. Even at these early developmental timepoints, the density of melanocytes is quite high (1000 cells/mm 2 ). The density increases further around the time of epidermal stratification (80–90 days' EGA) and initiation of appendageal development. Between 3 and 4 months EGA, depending on body site and the race of the fetus, melanin (visible pigment) production becomes detectable, and by 5 months, melanocytes begin transferring melanosomes to the keratinocytes, a process that will continue after birth. Although all melanocytes are in place at birth and melanogenesis is well under way, the skin of the newborn infant is not fully pigmented and will continue to darken over the first several months. This is most apparent in individuals with darker skin.
Langerhans' cells, the other major immigrant population, are detectable within the epidermis by 40 days' EGA. Similar to melanocytes, the early embryonic Langerhans' cells do not yet possess the specialized organelles characteristic of mature cells, but can be distinguished from other epidermal cells by their dendritic morphology, immunopositive reaction for the HLA-DR surface antigen, and high levels of ATPase activity. After the transition from embryo to fetus, they begin to express the CD1 antigen on their surface and to produce characteristic granules of mature Langerhans' cells. Although the extent of dendritic processes from individual Langerhans' cells increases during the second trimester, the total number of cells remains low and only increases to typical adult numbers in the third trimester.
Another distinct subset of cells within the basal cell layer are Merkel cells, which are highly innervated neuroendocrine cells involved in mechanoreception. Merkel cells can be round or dendritic, and are found at particularly high densities in volar skin. They are frequently associated with epidermal appendageal structures and are occasionally detected within the dermis. Their distinguishing morphologic and immunohistochemical features are cytoplasmic dense-core granules, keratin 18, and neuropeptide expression, which can be detected as early as 8–12 weeks' EGA in palmoplantar epidermis and at slightly later times in interfollicular skin. Recent keratin expression data, as well as transplant studies, suggest that Merkel cells are derived from pluripotent keratinocytes, rather than neural progenitors such as neural crest, but the results are not conclusive.
Many clinical defects are known to affect normal pigmentation. Defects in melanoblast migration, proliferation, and/or survival occur in several clinical syndromes, and many of the genetic mutations responsible for these defects have been identified (see Chapter 23 ). Failure of an adequate number of melanoblasts to completely supply distal points on their embryonic migration path occurs in the different types of Waardenburg syndrome, as well as in piebaldism, resulting in depigmented patches on the central forehead, central abdomen, and extremities. These defects are associated with mutations in several different genes, including genes encoding transcription factors, such as Pax3 and MITF, as well as membrane receptors and their ligands, such as endothelin 3, endothelin-receptor B, and c- kit . In albinism, however, melanocyte development is normal, but production of pigment or melanin is inadequate. The most severe form of oculocutaneous albinism results from null mutations in the gene encoding tyrosinase, the rate-limiting enzyme in the production of melanin. Less severe forms of albinism are caused by mutations in tyrosinase alleles, which lead to partial loss of function, as well as by mutations in other genes encoding proteins important in melanin assembly in melanosomes or transport.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here