Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Two predominant methods of blood propulsion are used: positive displacement roller pumps and constrained vortex centrifugal-type pumps.
Modern heart-lung machines are equipped with a number of alarm systems and redundant backup systems to overcome primary system failures.
Blood gas exchange devices have improved over time in terms of reduced blood-surface interface, improved efficiency, and improved blood device-related inflammatory response.
Gaseous and particulate microemboli enter the cardiopulmonary bypass (CPB) circuit from entrainment in the venous inflow to the circuit and also through the cardiotomy suction system. None of the currently available CPB systems remove all of the emboli.
Cardioplegia must be delivered accurately to prevent myocardial damage, and new pump delivery systems provide a better operator-interface for effective delivery.
Blood conservation is paramount, and an effective system involves proper equipment selection for the size of the patient, careful coagulation management, and the use of advanced techniques such as acute normovolemic hemodilution, retrograde and antegrade priming, ultrafiltration, and autotransfusion.
Extracorporeal membrane oxygenation (ECMO) has had a profound resurgence as therapy for acute cardiopulmonary failure.
Advancements in equipment and improvements in techniques and management have led to better outcomes for patients undergoing ECMO.
Venoarterial (VA) ECMO should be considered for patients with acute cardiac or combined cardiac and respiratory failure.
Venovenous (VV) ECMO is indicated for patients with adequate cardiac function in the setting of severe acute respiratory failure refractory to standard management.
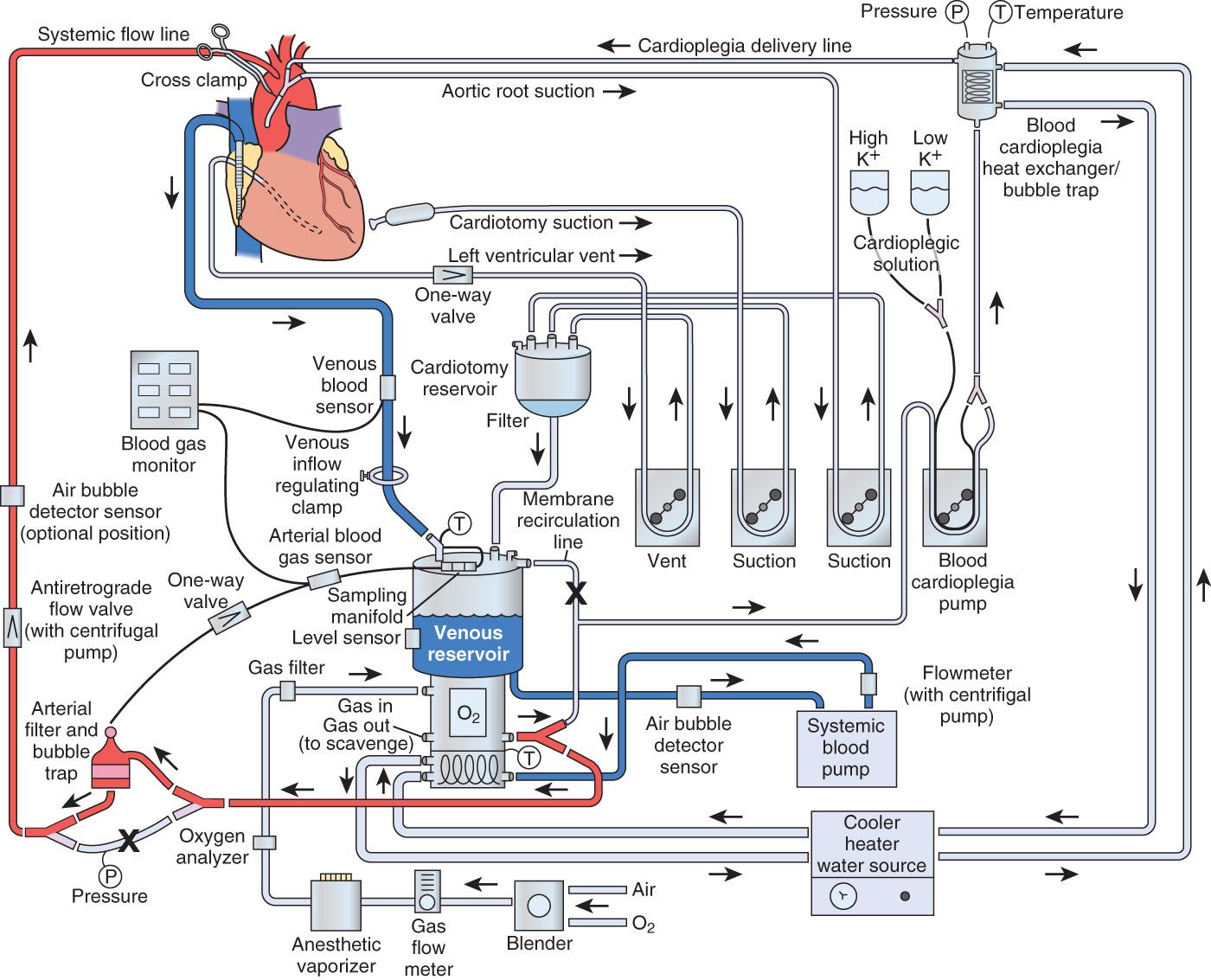
Since the 1950s, cardiopulmonary bypass (CPB) has undergone a dramatic metamorphosis from a lifesaving, yet life-threatening, technique to a procedure practiced nearly 1 million times a year throughout the world. It is uncommon in today's medical environment to encounter such an invasive procedure, with such significant risk and inherent morbidity, being practiced as routine. The goal of all techniques of CPB has always been to design an integrated system that could provide nutritive solutions with appropriate hemodynamic driving force to maintain whole-body homeostasis, without causing inherent injury.
All extracorporeal flow occurs through processes that incorporate a transfer of energy from mechanical forces to a perfusate and, ultimately, to the tissue. Most extracorporeal pumps fall into one of the following categories: positive displacement (PD) roller pumps or constrained vortex (centrifugal).
The PD pump operates by occluding tubing between a stationary raceway and rotating roller pumps ( Fig. 26.1 ). The pumping mechanism is also referred to as the pump head, and the tubing that traverses the raceway is referred to as the pump header . In a PD pump, fluid is displaced in a progressive fashion from suction to discharge, with the capacity of the displacement dependent both on the volume of the tubing occluded by the rollers and on the number of revolutions per minute (rpm) of the roller. All PD roller pumps (RPs) use the volume in the pump header, which is referred to as a flow constant and is specific to each size of tubing referred to by the internal diameter of tubing, for calculating the flow of the pump. This is displayed on a digital readout and is referred to as the output (flow) of the pump. It is measured in liters per minute ( Box 26.1 ).
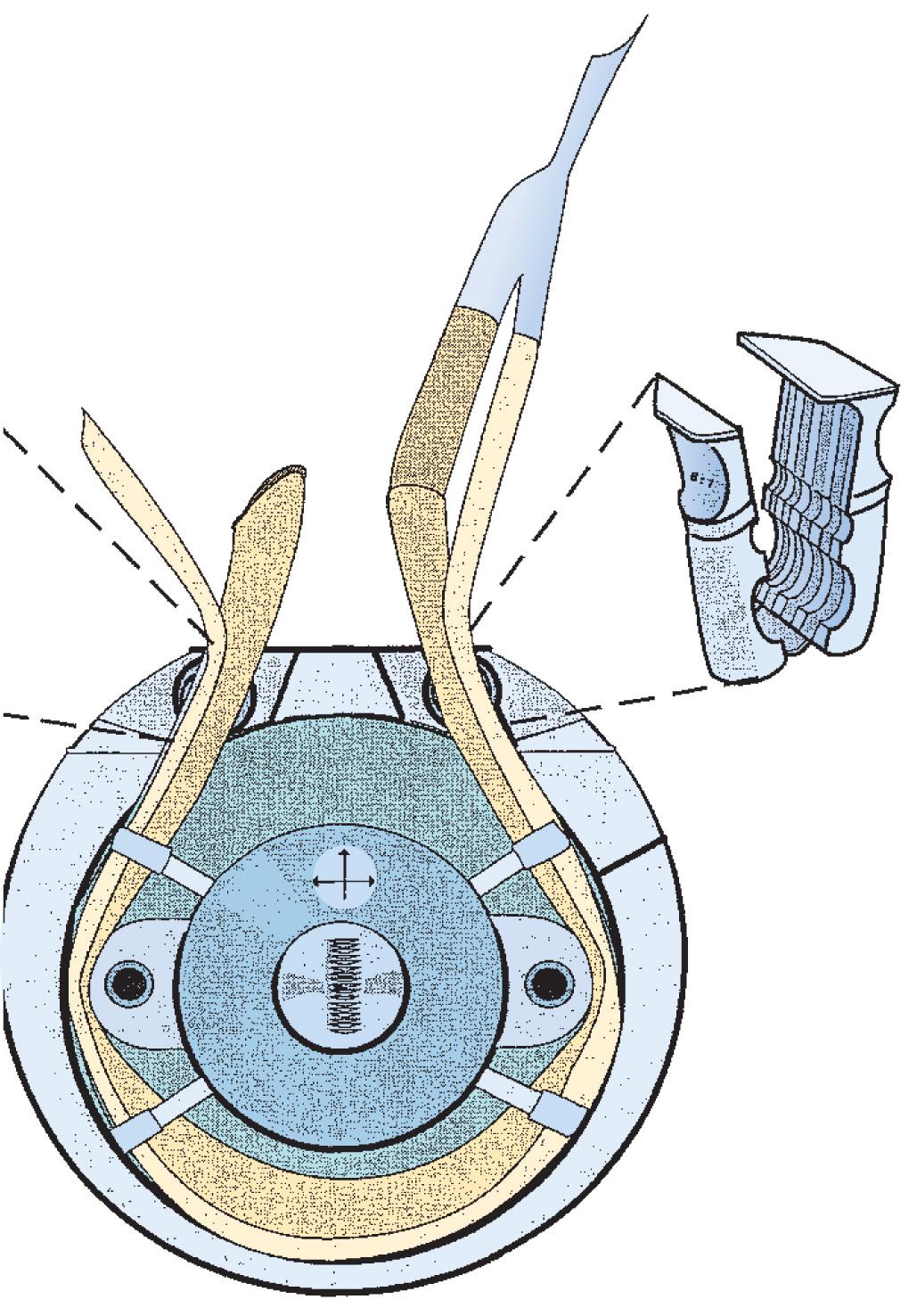
Composed of twin rollers
Deliver flow using positive displacement of the fluid in the tubing
Blood flow is calculated using tubing stroke volume and pump revolutions per minute
An underocclusive roller pump or an open shunt in the circuit may result in retrograde flow in the patient and in the cardiopulmonary bypass circuit
An overocclusive roller pump may increase hemolysis and produce spallation of the perfusion tubing
A modern heart-lung machine consists of between four and five of these RPs positioned on a base console ( Figs. 26.2 and 26.3 ). Most machines are modular in design, permitting the rapid change-out of a defective unit in the case of single-pump failure. Each pump is independently controlled by a rheostat that functions to regulate the rpm of the rollers. Each pump is calibrated according to specific flow constants that are calculated from the internal diameter of tubing, as well as the tubing length, placed in the pump raceway. Periodically, PD pumps are calibrated by performing a timed collection of pumped fluid to verify that after proper calibration the pump delivers the volume indicated on the pump flow display. Most of the hemolysis generated during a routine CPB procedure is not related to the occlusiveness of the arterial pump head but rather to the air-surface interface interaction occurring with the use of suction and “vent” line components of the circuit.

The second type of extracorporeal pump is a resistance-dependent pump termed a centrifugal pump (CP) or constrained vortex pump. The CP conducts fluid movement by the addition of kinetic energy to a fluid through the forced centrifugal rotation of an impeller or cone in a constrained housing ( Box 26.2 ). The greatest force, highest energy, is found at a point most distal to the center axis of rotation. CPs operate as pressure-sensitive pumps, with blood flow directly related to downstream resistance. Blood flow is, therefore, related to both the rpm of the cones or impellers and the total resistance.
Operate on the constrained vortex principle
Blood flow is inversely related to downstream resistance
Flow rate is determined using an ultrasonic flow meter
Increase in centrifugal pump revolutions per minute may result in heat generation and hemolysis
If the centrifugal pump is stopped, the line must be clamped to prevent retrograde flow
The acceptance of these devices in routine CPB has increased tremendously since first being introduced into clinical practice in 1969, and it is the pump of choice during emergency bypass procedures. The CP also has been used as a temporary ventricular assist device (VAD) because of its inherent safety features and pressure sensitivity, as well as relatively low cost.
Electromagnetic and Doppler ultrasonic flow meters are the two methods of measuring CP flow, as compared with the calculated flow display of the PD pumps, which is the product of a flow constant and the rpms. Some have argued that separate flow meters should be used with PD pumps to measure the flow directly to avoid errors that may occur related to an unocclusive roller head, open shunts in the circuit, or selection of the wrong flow constant.
Some of the most recent advances in pump design have been a result of a heightened awareness of increasing safety associated with complex operating systems. The PD pumps are pressure-independent, which means they will continue to pump regardless of downstream resistance . In a CPB circuit, the summation of resistances against which a pump must function includes the total tubing length, the oxygenator, the heat exchanger, the arterial line filter, the cannula, and the patient's systemic vascular resistance (SVR). Additional factors that influence SVR include the viscosity of the perfusate, related to the total formed element concentration, which primarily is dependent on the formed elements of blood and the temperature of the solution. Perfusionists routinely monitor the summation of all resistances and record this value as the arterial line, or system, pressure. Any acute change in resistance, such as unexpected clamping or kinking of the arterial line, results in an abrupt increase in arterial line pressure, which can lead to catastrophic line separation or circuit fracture anywhere on the high-pressure side of the circuit. A life-threatening event could occur on the initiation of CPB if the tip of the arterial cannula lodges against the wall of the aorta, undermining the intima of the vessel. Under these conditions, aortic dissection can occur as the vessel intima separates from the media, directing blood flow into a newly created false lumen. This dissection can extend throughout the entire length of the aorta. For this reason, perfusionists routinely check the line pressure after cannulation before the onset of CPB to ensure the presence of a pulsatile waveform, indicating proper cannula placement in the central lumen of the aorta. Either the absence of pulsatile pressure in the outflow portion of the perfusion circuit or an extremely high line pressure (>400 mm Hg when CPB is initiated) should immediately be investigated.
All heart-lung machines include a microprocessor-controlled safety interface with their pump consoles. These systems monitor and control pump function and serve as the primary mechanical safety control system for regulating extracorporeal flow. Pressure limits are set by the perfusionist and are determined by patient characteristics and the type of intervention performed. These units consist of early-warning alarms that alert the user to abrupt changes in pressure and will automatically turn off a pump when preset limits are exceeded. These safety devices have been used in both the main arterial pump and the cardioplegia pump; the latter become more important with the utilization of retrograde cardioplegia administration into the coronary sinus.
Electrical failure in the operating room (OR) can be especially catastrophic in the conduct of extracorporeal circulation (ECC) when the native heart and lungs are unable to function. When such an event occurs during CPB, it is imperative that instantaneous actions be instituted to minimize the risk for whole-body hypoperfusion. The perfusionist should be mindful of the power limitations of the electrical outlet used in the cardiac OR and also be aware of the location of the circuit breaker panel for the room and the specific number of the breaker in the panel for the outlet used for the heart-lung machine and other support equipment. Methods to ensure the safe conduct of CPB involve the incorporation of an emergency power source in the extracorporeal circuit that provides a secondary power source in the event of electrical interruption. Electrical failure during CPB was reported by 42.3% of respondents in a survey on perfusion accidents. Although hospitals are equipped with emergency generators for such events, their availability may be limited to certain electrical circuits within the operating suite. Furthermore, these emergency power systems require a brief interruption in power before a generator or backup source of power is initiated. Most heart-lung machines are equipped with uninterrupted backup power, sometimes referred to as the uninterrupted power source (UPS), whereby there is a seamless transfer from the wall power source to an internal battery within the pump should the wall power fail. Thus with this system, there is no loss of flow from the pumps that could result in retrograde flow and entrainment of air or disruption of settings and timers.
The ECC of blood incorporating total heart-lung bypass could not be accomplished were it not for the development of devices that could replace the function of the lungs in pulmonary gas exchange. The technology of pumps to replace the mechanical action of the heart was developed well before their incorporation in ECC. Therefore the limiting factor hindering the progression of CPB was the development of an artificial lung, or blood gas exchange device (BGED), commonly referred to as a membrane oxygenator ( Box 26.3 ). The term membrane denotes the separation of blood and gas phases by a semipermeable barrier, whereas oxygenator refers to the change in oxygen partial pressure that occurs by the arterialization of venous blood. However, “oxygenator” is a misrepresentation of the functional ability of these systems to perform ventilatory control of carbon dioxide. Numerous engineering challenges hindered the development of BGEDs, but two of the most pressing were the design of high-capacity units for gas exchange with low rates of bioreactivity. The latter requirement, also termed biocompatibility, was imperative to reduce both red blood cell (RBC) trauma and activation of the formed elements of blood.
Hollow-fiber membrane oxygenators are commonly used for cardiopulmonary bypass
An oxygen gas mixture flows through microporous polypropylene hollow fibers
Blood flow is directed over the microporous hollow fibers
Recently nonporous polymethylpentene (PMP) hollow fibers have been developed
PMP fibers provided a more durable surface for prolonged oxygenation such as extracorporeal membrane oxygenation
PMP fibers do not permit the passage of volatile anesthetics such as isoflurane
Membrane oxygenators are made of three distinct compartments: gas, blood, and water. The latter phase is also termed the heat exchange compartment and is used for temperature control. Gas and blood are partitioned into separate compartments with either a limited or absent gas-blood interface. Microporous membrane oxygenators have a blood-gas interface that is formed when the inner blood contact surface has been exposed to plasma; and a protein layer is deposited, acting as a diffusible barrier to gas exchange. The most common material in use today in membrane oxygenators is microporous polypropylene, which has excellent capacity for gas exchange and good biocompatibility. Despite the improvements made to extracorporeal devices over the past several decades, once blood is exposed to synthetic surfaces, hematologic changes result. Initially, complement is activated mainly through alternative pathways, resulting in the liberation of toxic mediators such as C3a and C5a. Both platelets and leukocytes that elicit a complex series of inflammatory and hemostatic reactions that ultimately increase the risk for postoperative complications are activated.
Gas transfer in membrane oxygenators is a function of several factors, including surface area, the partial pressures of venous oxygen and carbon dioxide, blood flow, ventilation flow (called sweep rate ), and gas flow composition. Membrane devices independently control arterial oxygen and carbon dioxide tensions (Pa o 2 and Pa co 2 ). Pa o 2 is a function of the F io 2 , whereas Pa co 2 is determined by the sweep rate of the ventilating gas. This independent control of ventilating gas results in arterial blood gas values more closely resembling normal physiologic blood gas status. However, it is common for perfusionists to maintain Pa o 2 levels in the 150- to 250-mm Hg range during CPB because of the limited reserve capacity of membrane oxygenators.
There are two general categories for venous reservoirs: open and closed systems ( Box 26.4 ). Open systems have a hard polycarbonate venous reservoir and usually incorporate a cardiotomy reservoir and defoaming compartment (see Fig. 26.2 ). Closed systems are collapsible polyvinylchloride bags that have a minimal surface area and often a thin single-layer screen filter, and they require a separate external cardiotomy reservoir for cardiotomy suction. The use of an open system offers several distinct advantages. Unlike collapsible reservoirs, it is not necessary to aspirate air actively, which may be entrained in the venous line during CPB. The large buoyant air migrates to the top of the reservoir and escapes through strategically placed vents on the reservoir cover.
Open systems have polycarbonate hard-shell reservoirs and are usually equipped with an integrated cardiotomy reservoir
With open systems, venous return may be improved by applying regulated suction to the reservoir (vacuum-assisted venous drainage)
With open systems, buoyant air bubbles escape to the atmosphere at the top of the reservoir
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here