Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
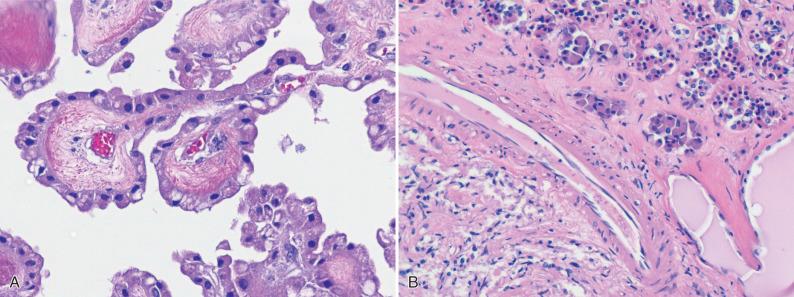
Epithelial structures are scarce in the normal central nervous system (CNS). Only the choroid plexus ( Fig. 16.1A ) and the pituitary gland's anterior and intermediate lobes contain true epithelial cells. The latter are derived from Rathke pouch epithelium that migrates to its place in the sella during early development ( Fig. 16.1B ). Thus, epithelial and epithelioid lesions that involve the brain include a mixture of tumors derived from these tissue types, as well as a much larger number of lesions derived from sources outside the CNS. This category includes several entities with topical overlap, some of which are covered elsewhere in this text. For instance, craniopharyngioma is discussed in Chapter 20 , whereas the choroid plexus tumors are covered in Chapter 8 . Instead, the current chapter covers four major topics: metastases, paragangliomas, papillary tumor of the pineal region (PTPR), and developmental cysts. Of these, metastatic neoplasms are by far the most commonly encountered. This chapter addresses both metastatic carcinomas and “epithelioid” metastases, such as melanoma and alveolar soft parts sarcoma, due to broadly overlapping histopathology. In contrast, other metastatic or secondary neoplasms, such as lymphomas and leukemias, germ cell tumors, and spindle cell sarcomas, among others, are covered in their respective chapters along with the primary CNS versions of these same neoplasms. Paragangliomas are neuroendocrine epithelioid tumors that affect the craniospinal axis in stereotypic locations. The PTPR has been included in this, rather than in Chapter 11 (Pineal Parenchymal Tumors) because, although its histogenesis and relation to the pineal gland has not been fully established, its epithelial histopathology is among its most striking features and this otherwise fits the patterns-based diagnostic approach emphasized in this text. Lastly, the great majority of developmental cysts are either epithelial or epithelioid and are therefore similarly discussed here.
Whereas systemic metastasis of primary CNS neoplasms is exceedingly rare, metastases to the brain are extremely common. In fact, metastatic CNS disease is far more common than primary neoplasia and is becoming even more prevalent with time. This is because the brain is a common site of end-stage failure for systemic cancers, where it represents a “safe haven” from chemotherapies that don't cross the blood-brain barrier. Nonetheless, the exact incidence of brain metastasis is difficult to determine. In fact, it is likely underestimated, given that asymptomatic patients do not routinely receive imaging and most autopsy series are outdated. The vast majority of systemic malignancies that reach the brain arrive by a hematogenous route, although retrograde perineurial spread involving cranial nerves occurs occasionally, particularly with head and neck mucosal and salivary gland malignancies. Direct spread from the adjacent bones of the craniospinal vault also occurs.
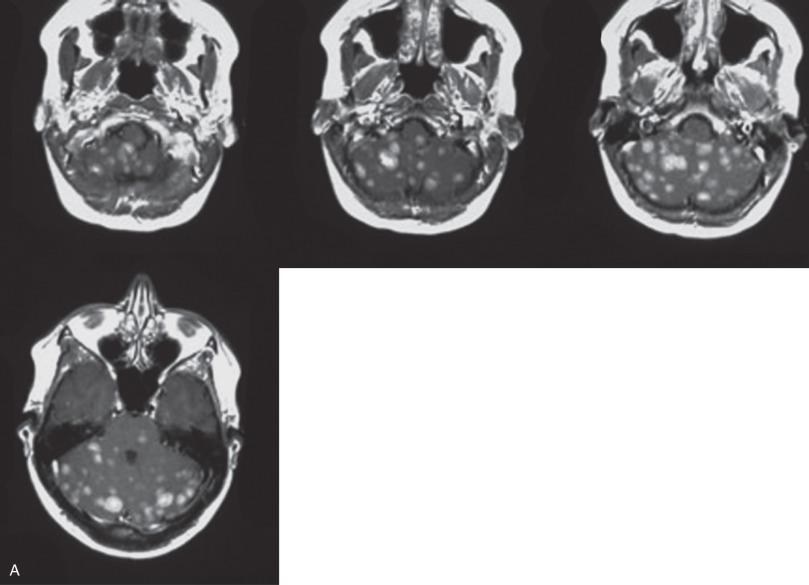
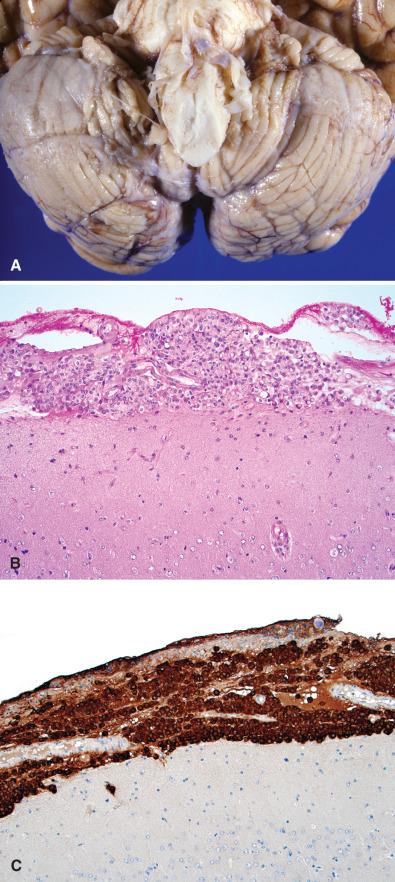
The most common systemic primary sites, in order of frequency, are lung, breast, skin (melanoma), kidney, and the gastrointestinal tract. Most nonpulmonary cancers first metastasize to the lungs before reaching the brain. Although these lung metastases can remain clinically silent, the brain masses typically cause symptoms, prompting clinical attention. Less commonly, metastasis to the CNS can bypass the lungs, especially those reaching the posterior fossa. The number of metastatic foci ranges widely, from solitary to miliary. Primary tumors vary in their tendency to produce single versus multiple metastases depending on their site of origin. For example, lung carcinoma (especially small cell), breast carcinoma, and melanoma typically produce multiple metastases, whereas metastatic colorectal carcinoma is solitary in about 50% of cases. For renal cell carcinoma (RCC), solitary metastasis is more common than multiple metastases. The most common primary source of miliary metastasis (“carcinomatous encephalitis”) is lung ( Fig. 16.2 ). Similarly, the most common primaries associated with spread through the cerebrospinal fluid (CSF) or “meningeal carcinomatosis” (“carcinomatous meningitis”) are lung and breast cancers. Although metastases may occur at virtually any age, the vast majority are in older adults.
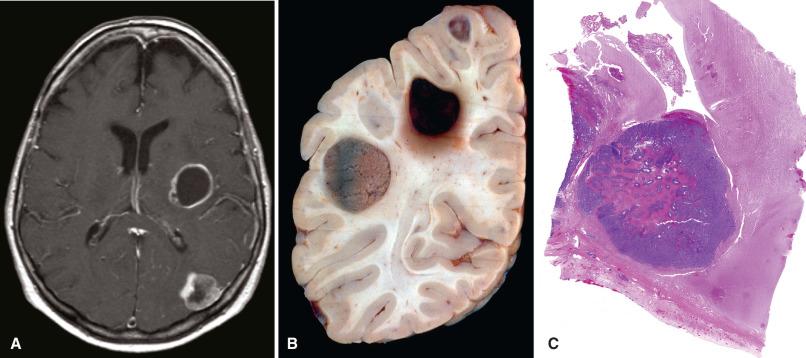
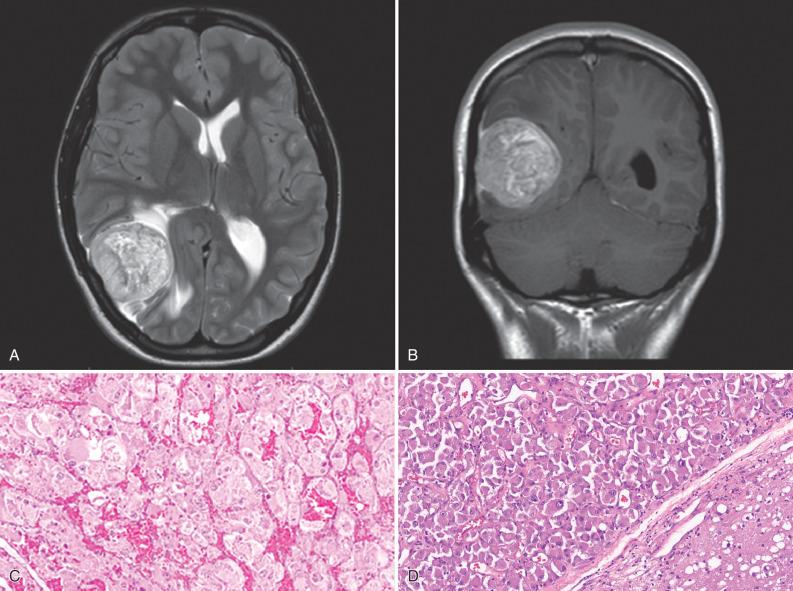
Metastatic disease can involve any CNS compartment, including the bones of the skull and spine, meninges (leptomeninges or dura), subarachnoid space (“carcinomatous meningitis”), and CNS parenchyma (brain much more common than spinal cord). Since metastases to the brain travel a hematogenous route and the middle cerebral artery (MCA) has the straightest, least impeded path to the brain, the MCA territory is the most commonly afflicted. Moreover, cerebral blood vessels often branch acutely and have reduced diameters at the corticomedullary junction, essentially forming microscopic filters that trap intravascular clusters of tumor cells. Thus, the interface of cortex and white matter is a preferential site for metastases. The vast majority of metastases present as sharply demarcated, contrast-enhancing masses, with or without discernable foci of necrosis ( Fig. 16.3 ). Nonetheless, any site can be involved, and some primary sites have a predilection for specific compartments. Prostate, breast, kidney, thyroid, and lung carcinomas often metastasize to bone (remembered using the mnemonic “lead kettle” or “PB-KTL”). Breast carcinoma frequently gives rise to dural and leptomeningeal metastases. For unknown reasons, RCC is the most frequent type of metastasis to the choroid plexus. Hemorrhagic metastases are seen most often with metastatic RCC, melanoma, choriocarcinoma ( Fig. 16.4 ), and lung carcinoma.
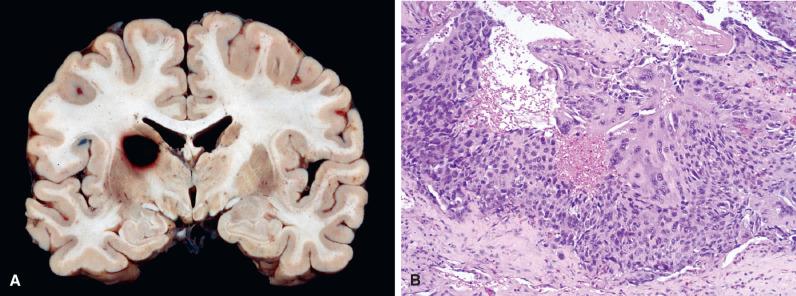
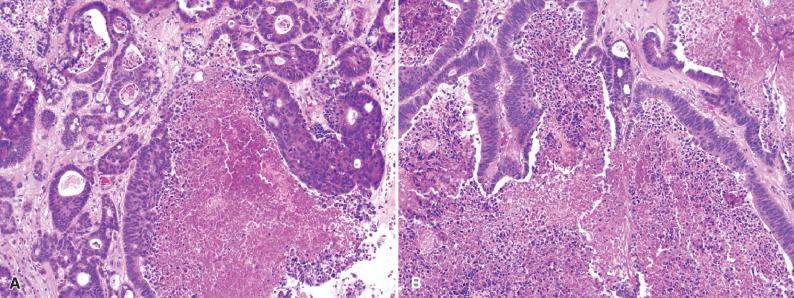
Meningeal carcinomatosis (or “melanomatosis,” “lymphomatosis,” “gliomatosis,” etc.) represents subarachnoid dissemination with widespread meningeal studding ( Fig. 16.5 ). Radiologically, this manifests as diffuse or multifocal leptomeningeal enhancement, while grossly, the involved meninges appear cloudy or opaque. CSF cytology is often positive for malignant cells in these patients.
Metastases to the CNS may retain histopathologic features of the primary tumor or may be poorly differentiated. A prior history of systemic malignancy or classic morphologic features often leads to a straightforward diagnosis, although poorly differentiated neoplasms and lack of a prior history are relatively common, with the “metastasis of unknown primary” accounting for roughly 10% to 20% of cases in surgical series. In such situations, ancillary studies, especially immunohistochemistry, can be extremely helpful in narrowing the search for a primary. The pathologist was once limited to cytokeratin cocktails and epithelial membrane antigen (EMA) for carcinoma, carcinoembryonic antigen (CEA) for gastrointestinal and pulmonary carcinomas, mucin stains to establish adenocarcinoma, and S-100 protein for melanoma. Although these broad-category stains remain useful, many more specific markers have been developed over the last couple of decades. A full discussion of all possible CNS metastases, their morphologic spectra, and the most useful ancillary studies is beyond the scope of this text; the interested reader is referred to two particularly useful reviews for greater detail. Nonetheless, the most common immunoprofiles associated with epithelioid metastases to the CNS are summarized in Fig. 16.6 , with common histopathologic and immunohistochemical patterns illustrated in Figs. 16.7 through 16.12 .
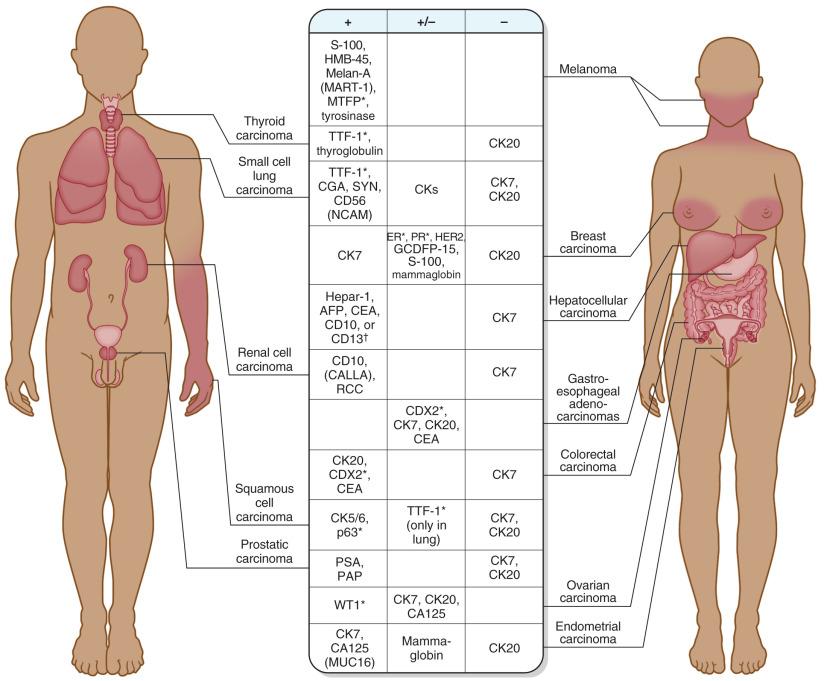
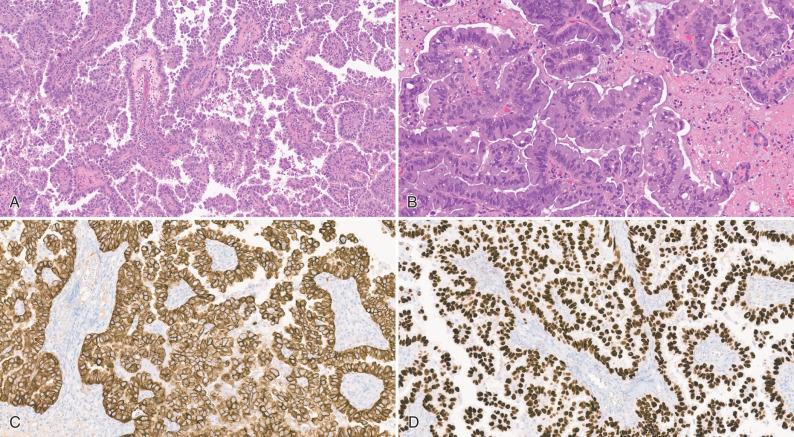
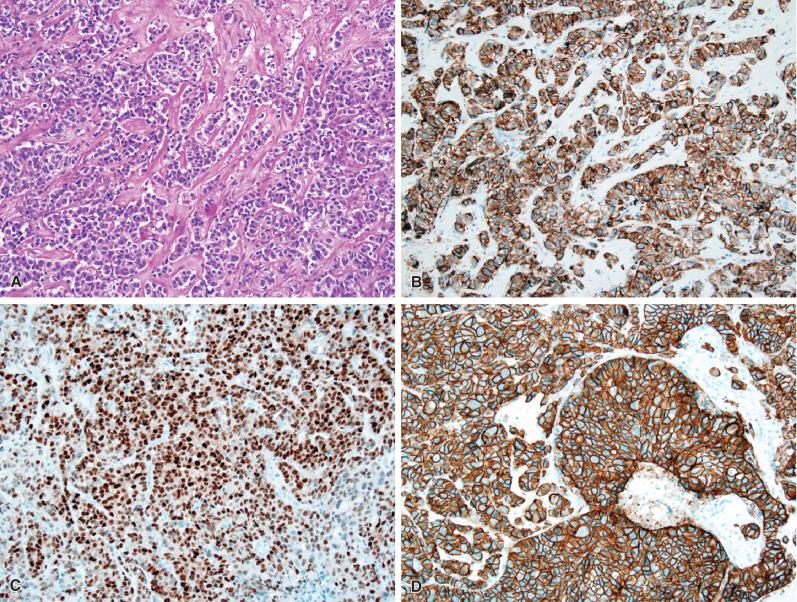
As mentioned, pulmonary cancers are the most common primaries to metastasize to the CNS. Until rather recently, lung carcinoma was divided into two basic categories for treatment purposes: small cell and non-small cell, with little importance attached to the specific histopathologic subtype of non-small-cell carcinoma. However, recent advances in targeted chemotherapy have highlighted the importance of distinguishing adenocarcinoma from squamous cell carcinoma, because a diagnosis of the former will initiate mutation testing at many institutions for activating EGFR gene mutations, as well as for negative predictors of response such as mutations in KRAS, BRAF, and HER2 (ERBB2). It is common for patients to present for neurosurgical resection of a metastatic lesion with a clinical history of “non-small-cell carcinoma” that has been based on pulmonary fine-needle aspiration. In other instances, the diagnosis of “lung cancer” may be established based solely on imaging of the lung, either before or after the resection of the brain metastasis. In such situations, it is incumbent on the pathologist to provide a definitive assessment of the CNS resection specimen so that molecular testing can be performed if the final diagnosis is adenocarcinoma. In cases in which the morphologic features are ambiguous on hematoxylin and eosin staining, mucin stains and immunophenotyping may be of assistance (see Fig. 16.6 ). The combination of CK7, CK20, and TTF-1 is particularly useful. The majority of adenocarcinomas and poorly differentiated non-small-cell lung carcinomas show strong diffuse positivity for CK7 and TTF-1 (see Fig. 16.7 ), with CK20 being negative. However, there are important exceptions. For example, “bronchioalveolar carcinomas” (also called “lepidic predominant adenocarcinomas”) may be positive for all three markers. Additionally, most adenocarcinomas are positive for Napsin A. Most small-cell carcinomas are diffusely positive for TTF-1, but negative for both CK7 and CK20, while also expressing a variety of neuroendocrine markers (see Fig. 16.8 ). In contrast to adenocarcinomas, squamous cell carcinomas typically show strong cytoplasmic positivity for cytokeratins 5 and 6 and strong nuclear positivity for p63 and p40 proteins. They are usually negative or only weakly positive for TTF-1. CK7 and CK20 are similarly negative in most examples.
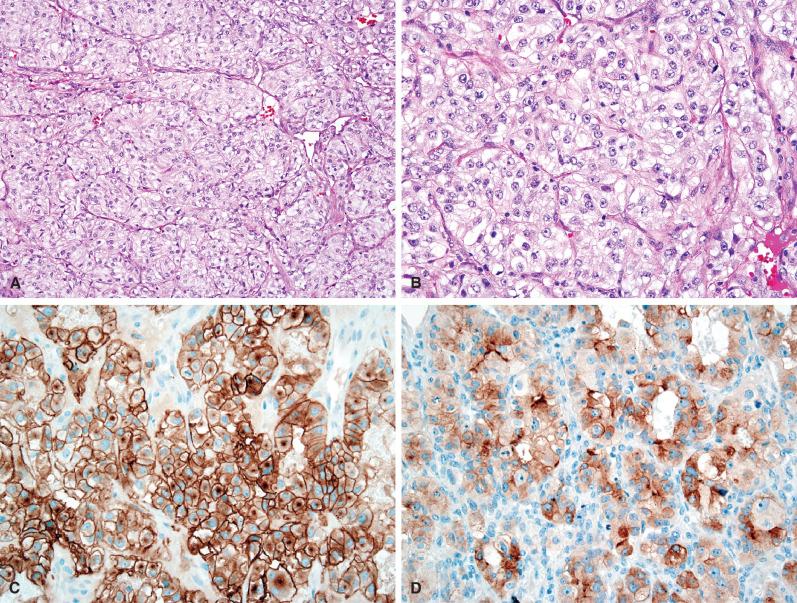
Brain metastases from the breast are most frequently derived from ductal carcinomas (see Fig. 16.9 ). Similar to lung adenocarcinomas, the majority of breast carcinomas are strongly CK7 positive and CK20 negative. However, they do not express TTF-1. Instead, there are subsets that variably express other diagnostically useful markers such as estrogen receptor, progesterone receptor, HER2, gross cystic disease fluid protein 15 (GCDFP-15), and mammaglobin, as well as GATA3. Hormone stains and HER2 status by immunohistochemistry, along with HER2 fluorescence in situ hybridization (FISH) studies, may provide prognostic and predictive information for targeted therapies. Although these tests may have been performed on the primary neoplasm, analyses of the metastasis can still be helpful since the status of markers occasionally changes, presumably as a minor clone becomes dominant in the metastasis.
Other particularly useful markers include CD10 (common acute lymphoblastic leukemia antigen, or CALLA) and RCC for renal cell carcinomas (see Fig. 16.10 ), CK20 and CDX2 for colorectal carcinomas (see Fig. 16.11 ), and a growing list of useful melanoma markers (see Fig. 16.12 ; see also Chapter 19 ). S-100 protein remains among the most sensitive markers for melanoma, being expressed in even sarcomatoid examples. However, it is relatively nonspecific, given its expression in the majority of gliomas, nerve sheath tumors, a variety of sarcomas, and occasional carcinomas, such as breast. An additional caveat regarding the use of S-100 protein is that a false negative result is very common on previously frozen tissue. Less sensitive, but much more specific are Melan-A (melanoma antigen recognized by T cells 1, or MART-1) and HMB-45, with newer markers including microphthalmia transcription factor (MITF) and tyrosinase. The use of a melanoma-screening cocktail (e.g., a cocktail including HMB-45, Melan-A, and tyrosinase) has the added advantage of including multiple individual antibodies, thereby minimizing tissue usage when material is limited.
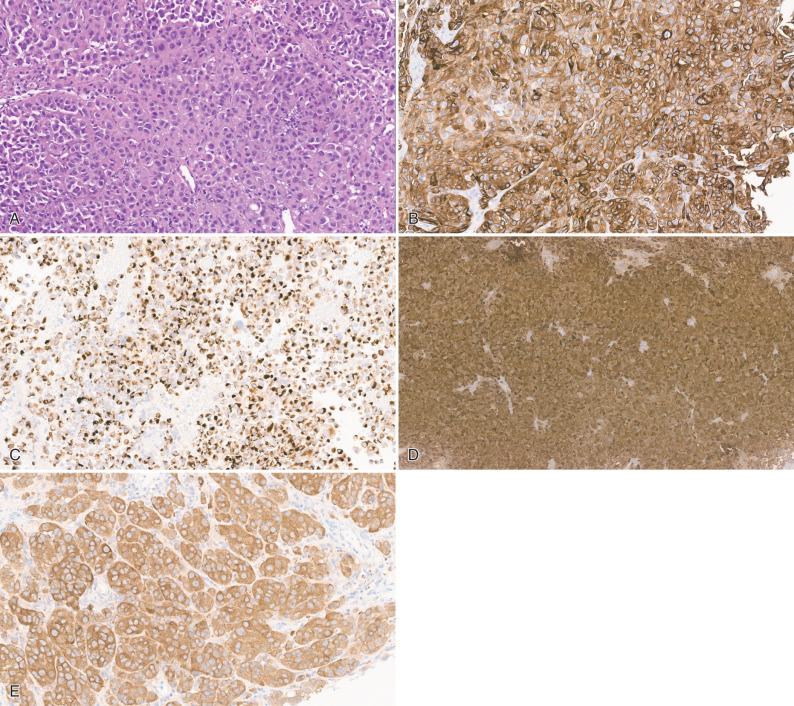
Many other epithelioid neoplasms can metastasize to the CNS, albeit much less commonly (see Fig. 16.6 ). Although quite rare, metastatic choriocarcinoma (see Fig. 16.4 ) warrants comment because of its predilection for young patients, acutely life-threatening nature due to its marked tendency to bleed, and responsiveness to chemotherapy. This germ cell tumor has a particularly high affinity for the brain and occasionally manifests as a brain metastasis of unknown primary. A recent history of pregnancy may be a clue to the gestational form in women. Similarly, metastatic sarcomas to the brain are nearly always late complications, so that the primary is already well established. However, alveolar soft parts sarcoma (ASPS) represents a rare epithelioid subtype with a known predilection for adolescents and young adults; presentation as a CNS metastasis of unknown primary occurs in a significant subset of cases ( Fig. 16.13 ). Although the histogenesis remains elusive, epithelial markers are negative and cytoplasmic periodic acid–Schiff (PAS)–positive crystalline inclusions are diagnostically useful. Also, detection of TFE3 expression by immunohistochemistry or testing for the presence of the fusion transcript ASPL-TFE3, resulting from the t(X;17)(p11.2;q25) translocation, can confirm the diagnosis.
Tumor-to-tumor metastasis most commonly involves the metastasis of a common and rapidly growing malignant systemic cancer, such as lung carcinoma, breast carcinoma, or melanoma, to a slowly growing, indolent primary CNS tumor, such as pituitary adenoma or meningioma. The latter is considered the most common host tumor for a metastasis, with breast carcinoma being a particularly common primary metastatic source. Since both tumor types are hormonally related tumors in women, some have speculated about the potential for shared risk factors.
Rarer combinations of primary and recipient tumor also usually follow the general principle of a malignant neoplasm metastasizing to an indolent one, such as a reported case of melanoma metastatic to central neurocytoma.
The differential between one metastatic neoplasm and another is discussed in the prior section on Histopathology and Ancillary Studies. However, a differential diagnosis with primary CNS neoplasms is also common. For example, in older adults with poorly differentiated metastases, the possibility of glioblastoma or other high-grade glioma is often considered. Conversely, glioblastomas of predominantly epithelioid or rhabdoid morphology can also be problematic and mimic metastatic carcinoma, melanoma, or sarcoma. Similarly, anaplastic oligodendrogliomas may appear remarkably epithelioid or carcinoma-like. In large resections, this differential is rarely a problem, since the interface with adjacent brain demonstrates a diffusely infiltrative growth pattern for glioblastoma and sharp demarcation in the case of a metastasis. A neurofilament protein (NFP) immunostain can further highlight this difference by showing entrapped axons in the former and axons pushed to the periphery in the latter. Epithelioid glioblastoma and pleomorphic xanthoastrocytoma (PXA) may be considered in the differential diagnosis of epithelioid CNS lesions. These two primary CNS tumors are frequently positive for mutations in the BRAF gene (i.e., BRAF p.V600E), similar to metastatic melanoma ( Box 16.1 ).
Metastatic melanoma
Epithelioid glioblastoma
Pleomorphic xanthoastrocytoma
Papillary craniopharyngioma
The interface between tumor and brain may not be evident in smaller biopsy specimens that are composed predominantly of tumor, and, in addition, occasional metastases demonstrate limited degrees of glioma-like brain invasion. In such situations, immunohistochemistry is valuable. Most carcinomas are strongly positive for cytokeratin, whereas glioblastomas express glial fibrillary acidic protein (GFAP), as well as transcription factors, such as OLIG2 and SOX10. However, an important pitfall is that most broad-spectrum cocktails, such as AE1/AE3, cross-react with GFAP, with positivity causing diagnostic confusion for the uninitiated. Thus, in the CNS, it is preferable to start with a low-molecular-weight cytokeratin mix, such as CAM 5.2. If the tumor is positive, more specific cytokeratins (CK7, CK20, CK5/6) may be of additional utility. It is also important to remember that S-100 protein is commonly positive in both gliomas and metastatic melanoma, so the more specific melanocytic markers are required for this differential diagnosis.
Primary CNS lymphoma (PCNSL) is another high-grade primary brain tumor that often enters the differential diagnosis of a contrast-enhancing mass lesion in an older adult patient. A common problem is a small open or stereotactic biopsy that yields a small amount of tissue showing a large-cell malignant neoplasm with necrosis and no appreciable brain interface. The principal entities in the differential diagnosis would be metastatic carcinoma, glioblastoma, and large B cell PCNSL. A helpful immunohistochemical panel in such cases is GFAP, CAM 5.2, and CD20 (or a lymphoma cocktail containing CD20, CD3 or CD5, and CD45). A significant number of PCNSL have been shown to have a Leu265Pro mutation in the MYD88 gene, the same mutation frequently present in patients with Waldenström macroglobulinemia (WM). Testing for the MYD88L265P mutation can help establish a diagnosis of lymphoma versus a reactive lymphocytic proliferation. Inhibitors of kinases in the MYD88 signaling pathway are commercially available for the treatment of WM, but their use in the treatment of MYD88L265P-positive PCNSL remains to be explored. Anaplastic ependymomas and choroid plexus carcinomas may rarely enter into the differential diagnosis with metastasis as both may display relatively sharp brain–tumor interfaces. Nevertheless, the clinical presentation is quite different in the vast majority of such cases, given that most intracranial ependymomas present in children, whereas choroid plexus carcinomas are almost exclusively seen in infants. Although rare, both of these tumor types can be encountered in adults, such that immunohistochemistry and electron microscopy may be useful (see Chapter 8 for greater detail). In contrast to the vast majority of epithelioid metastases, most ependymomas are strongly GFAP positive and cytokeratin negative. EMA is often positive, but displays scattered foci of cytoplasmic dot-like and rare membrane pattern staining, rather than the strong widespread positivity typical of carcinomas. The majority (~70%) of supratentorial ependymomas have a characteristic genetic rearrangement involving the fusion of C11orf95 (a poorly characterized open reading frame on chromosome 11) and RELA (a subunit of the NF-κB complex). The C11orf95-RELA fusion drives oncogenesis and serves as a diagnostic marker. Ependymoma, RELA fusion positive was officialy codified in the 2016 World Health Organization (WHO) classification. Expression of L1 cell adhesion molecule (L1CAM) correlates with the presence of the RELA fusion and might serve as a surrogate marker for detection of the genetic rearrangement. Most choroid plexus carcinomas are strongly cytokeratin positive, but are EMA negative or show only limited positivity. Transthyretin is positive in the majority of choroid plexus tumors, but is less specific than originally thought. Potentially useful new markers include Kir7.1 and stanniocalcin, although these are not widely available. Expression of the inward rectifier potassium channel Kir7.1 has been detected in normal choroid plexus and choroid plexus papilloma, but thus far has not been detected in other primary brain tumors. The glutamate transporter EAAT1 could help in differentiating between neoplastic and non-neoplastic choroid plexus, as expression of EAAT1 is rare in non-neoplastic choroid plexus. Additionally, many choroid plexus carcinomas express GFAP at least focally.
The differential of dural or intraventricular metastases often includes anaplastic meningioma. Finding lower-grade foci of classic meningioma is helpful, but not present in every case. Unfortunately, no absolutely specific meningothelial markers are available, and high-grade meningiomas may express cytokeratins, including CK7. The most commonly utilized meningioma marker is EMA, yet this has only limited use in this differential since most carcinomas express it even more widely and strongly than meningiomas. Although even less specific overall, nearly all meningiomas are strongly and diffusely positive for vimentin, unlike the majority of metastatic carcinomas. However, most RCCs and melanomas also express vimentin, so more specific markers of these two tumor types should be added when they are viable considerations. Immunohistochemistry for somatostatin receptor 2a (SSTR2a) offers additional utility, being strongly and extensively positive in even the most poorly differentiated meningiomas. This marker is also expressed in many neuroendocrine neoplasms, but is typically negative or weak in metastatic nonendocrine carcinomas. In the vast majority of cases, the specific markers listed in Fig. 16.6 establish a metastatic malignancy. However, in rare cases where even these studies are equivocal, either electron microscopy or molecular studies can be useful for demonstrating meningioma-specific ultrastructural or genetic features and thus establishing a definitive diagnosis (see Chapter 13 for greater detail).
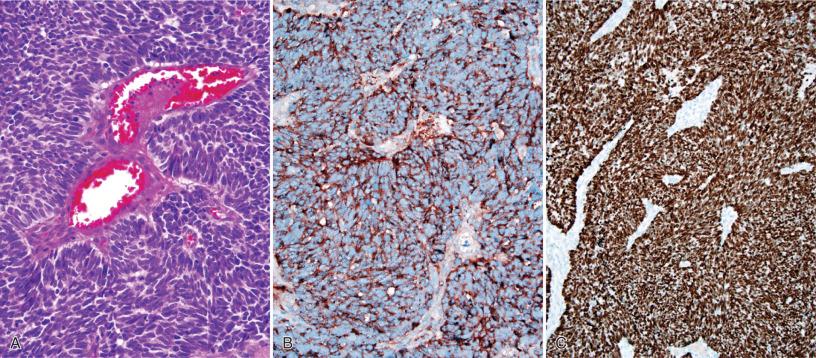
Another relatively common differential diagnosis, especially in the posterior fossa, is that of metastatic RCC versus hemangioblastoma ( Fig. 16.14 ); this can be particularly challenging if the patient has von Hippel-Lindau syndrome and is predisposed to both clear cell tumors. In most examples, this distinction is evident on routine histology, since hemangioblastomas have foamier cytoplasm and darker nuclei with degenerative-type atypia, whereas RCCs often have large vesicular nuclei with prominent nucleoli, along with other high-grade features, such as necrosis and mitotic activity. Nevertheless, in some cases the distinction is difficult. RCCs are typically positive for cytokeratins, EMA, CD10, and RCC, whereas hemangioblastomas are positive for inhibin alpha, D2-40, neuron-specific enolase, and S-100 protein.
Genetic features of metastastic epithelioid neoplasms are essentially those of the primary tumor type, and a detailed discussion is beyond the scope of this text. As mentioned earlier, however, genetic testing in metastatic carcinomas is becoming more common as targeted forms of therapy are advanced, particularly in breast carcinomas and pulmonary adenocarcinomas. Some of these, as well as other novel therapies, are significantly improving the otherwise grim prognosis for patients with metastatic carcinomas and melanomas. As such, it is anticipated that ancillary testing in metastases will likely increase over time. Additionally, it is important to consider more therapeutically responsive epithelioid metastases in the differential diagnosis, such as germ cell tumors, particularly in younger patients. For the vast majority of patients, however, current therapy consists primarily of surgical resection or radiosurgery (e.g., Gamma Knife), or both for a single (or a few) metastasis, which seems to provide some survival benefit as well as relief of impending herniation for those with considerable mass effects. Chemotherapy is often a challenge, given the limited penetration of the blood-brain barrier by many of the currently available agents. Nevertheless, some drugs provide both systemic and CNS benefits. For patients with numerous widespread metastases or tumors known to disseminate widely, such as small-cell carcinoma, whole brain irradiation is the most common form of initial therapy. Patients with meningeal carcinomatosis have a particularly poor prognosis, and therapy often includes intrathecal chemotherapy and craniospinal irradiation.
Paragangliomas are distinctive neuroendocrine neoplasms that arise from the autonomic nervous system (“paraganglia”). In surgical neuropathology (tissue resected by a neurosurgeon), they are primarily encountered either in the filum terminale or in the jugulotympanic region, where they are thought to arise from the specialized chemoreceptor cells of the glomus jugulare. Because of this anatomic relationship, clinicians sometimes refer to them loosely as “glomus tumors,” but since they have no relationship to the pathologically defined vascular glomus tumors, the more accurate terminology is jugulotympanic paraganglioma. They also arise in paraspinal ganglia and the carotid bodies, but are more likely to be encountered by general pathologists in these locations. Paragangliomas are composed mostly of chief cells, with variable numbers of supporting sustentacular cells. In some tumors, scattered cells differentiate into ganglion cells, qualifying for the designation gangliocytic paraganglioma. Chemodectoma represents a common synonym, although this terminology is most often utilized with carotid body paragangliomas. The term pheochromocytoma is used exclusively for histologically identical tumors arising from the adrenal medulla. However, they are important to consider in the patient's past medical history, since hereditary disorders predisposing to paragangliomas such as type II multiple endocrine neoplasia, von Hippel-Lindau disease, neurofibromatosis type 1, and familial paraganglioma syndrome typically predispose to pheochromocytomas as well, although paraspinal or head and neck tumors are more common (also see Chapter 22 ).
Paraganglioma of the filum terminale (PFT) is a rare tumor and accounts for only 3% to 4% of all tumors arising in the cauda equina region. Nevertheless, it is a well-documented entity, with more than 200 reported cases in the literature. PFT has been reported in all age groups, from the first decade through the eighth, but is primarily a tumor of middle-aged adults (mean age around 45 years). There is a slight male predominance. Jugulotympanic paraganglioma (JTP) is similarly rare and afflicts young to middle-aged adults, but has a female predilection.
Because of its origin from the filum and the cauda equina region, PFTs usually manifest with low back pain and radicular symptoms. JTPs manifest with pulsatile tinnitus, conductive hearing loss, or lower cranial nerve palsies. In comparison to pheochromocytomas and paraspinal paragangliomas, manifestations of catecholamine secretion (labile hypertension, facial flushing, etc.), multifocality, familial inheritance, and signs of malignancy are relatively rare.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here