Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Management of aortic dissection is complex and directed by multiple patient and disease-specific factors requiring a complication-specific approach. Appropriate aortic imaging is paramount for both diagnosis and treatment planning. Initial blood pressure, heart rate control, and monitoring in the intensive care unit setting are critical. Descending aortic dissections (Stanford type B, DeBakey III) complicated by rupture, rapid false lumen expansion, tissue malperfusion, or intractable pain or uncontrollable hypertension are likely to require acute intervention. Conversely, uncomplicated type B dissections are typically treated medically. With the advent and evolution of endovascular techniques, less invasive treatment options are becoming available to the clinician more often. Ultimately, treatment of aortic dissection will likely employ multiple techniques and patient- and disease-specific approaches.
Appropriate treatment for an aortic dissection is determined by a combination of location and extent of the dissection, time from onset of symptoms, comorbid risk factors, and dissection-related conditions such as visceral malperfusion or impending rupture. Although most patients present with acute dissection, up to a third present with symptoms of more than 2 weeks’ duration. This distinction between acute and chronic dissection is of therapeutic significance because patients with acute aortic dissections are at highest risk for life-threatening complications. In the current era, treatment options can be divided into three main categories: medical management, surgical intervention, and endovascular intervention. Recommendations for the management of thoracic aortic dissection have been made by the 2010 American College of Cardiology Foundation/American Heart Association task force for the diagnosis and management of patients with thoracic aortic disease.
Ascending aortic dissections (Stanford type A, DeBakey I, II) account for about 60% of all dissections and are at high risk of acute complications including cardiac tamponade (5%), myocardial ischemia (7%–19%), acute aortic regurgitation (45%), heart failure (5%), stroke (8%), and, ultimately, death. The mortality rate for acute type A dissections without intervention is estimated at 1% per hour after patients arrive in the hospital, with mortality rates of 38%, 50%, 70%, and 90% at 24 hours, 48 hours, 1 week, and 2 weeks, respectively. In general, type A dissections require emergent surgical repair. Presently, there are no US Food and Drug Administration-approved endovascular devices for the ascending aorta.
There remains some controversy over the treatment strategy of type B aortic dissections. In 1965 DeBakey et al. published their study of a large series of 179 aortic dissection patients treated surgically, with an operative mortality of 21% and a 5-year mortality of 50%. In comparison with large series of patients with aortic dissections who had undergone nonoperative treatment, DeBakey et al. concluded that all patients with aortic dissections should undergo surgical intervention. Later, Wheat et al. advocated a selective approach based on the observation that surgical intervention carried a 25% early mortality, whereas patients treated pharmacologically had a 16% early mortality. It was not until 1970 when Daily et al. at Stanford introduced the Stanford dissection classification that the importance of distinguishing ascending and descending aortic dissections became apparent. It was widely held that type B dissections ought to be treated medically unless life-threatening complications were present. Although operative mortality for type B dissections significantly improved over time (57%–13% in the Stanford series), several risk factors, including visceral ischemia, aortic rupture, and older age, portended dramatically increased risk with surgical repair. In the current era of endovascular stent-grafts, it seems intuitive that less invasive techniques for stabilizing complicated acute type B dissections, which carry a high risk of mortality with and without surgical intervention, could provide an opportunity to improve outcomes in this very ill patient population.
Although 20% to 50% of uncomplicated type B aortic dissections demonstrate eventual aneurysmal dilatation within 3–5 years of diagnosis, there is a small risk of rupture in the acute setting, with a 30-day mortality of about 10% in contemporary studies. Most authors advocate medical treatment with blood pressure control and close follow-up with interval imaging. Many of these patients will ultimately require intervention beyond the acute setting, so some authors have suggested that asymptomatic descending aortic dissections be treated with stent-grafts to prevent late complications. However, long-term data comparing medical treatment and stent-grafts in uncomplicated acute type B dissections are insufficient at present to define the appropriate treatment strategy. The INSTEAD trial (Investigation of Stent-Grafts in Aortic Dissection) attempted to answer a similar debate in subacute and chronic type B dissections (within 2–52 weeks of onset) by randomizing patients to either medical therapy or stent-graft placement. The trial found no difference in all-cause and aorta-related mortality at 1-year follow-up, but 91% of those in the stent-graft group demonstrated evidence of aortic remodeling, compared with only 19% of those in the medical group. Even though the INSTEAD trial did not include acute aortic dissections (<2 weeks from onset), the trial did reveal a potential for stent-grafts to promote aortic remodeling and potentially prevent late aneurysmal degeneration. However, it is important to note that based on this trial, it appears that stent-graft treatment of patients with subacute or chronic aortic dissection offers no benefit in terms of reducing the risk of aortic rupture or enhancing life expectancy. Longer follow-up is necessary to understand the true benefit or lack thereof for stent-grafts in asymptomatic type B dissections.
In contradistinction, complicated type B aortic dissections, defined by the presence of visceral or peripheral malperfusion, rupture, rapid false lumen expansion, persistent pain, or uncontrollable hypertension, often require intervention. Despite significant improvement in operative mortality for acute type B dissections, operative mortality in the presence of visceral ischemia and rupture remains as high as 70%–80%. Addition of endovascular interventions has provided an opportunity for a less invasive, more expeditious procedure to stabilize the dissection, prevent rupture, and restore true lumen perfusion.
Endovascular treatment options include aortic stent-graft placement, dissection flap fenestration, and branch vessel stenting. Each technique aims to achieve one or both of the two major goals in treating acute aortic dissection: prevent aortic rupture and restore end-organ perfusion. Current indications for intervention encompass the life-threatening complications of acute aortic dissection, hence the term “complicated.” As mentioned previously, complicated dissections are defined by visceral or limb malperfusion, aortic rupture or impending rupture (e.g., rapid false lumen expansion), or evidence of an unstable dissection plane (e.g., persistent pain, uncontrollable hypertension).
In type A dissection, aortic branch vessel obstruction is usually corrected concomitantly with surgical management and repair of the ascending aorta. In type B dissection, branch vessel malperfusion is an indication for intervention. Recently, stent-graft placement over the primary entry tear has been increasingly performed as an alternative to open surgical distal fenestration of the intimal flap, percutaneous radiologic balloon fenestration of the aortic septum, operative replacement of the diseased aorta, or bypass graft reperfusion of ischemic vessels.
The concept of endovascular stent-graft repair is predicated on successful device placement over the primary entry tear to obliterate blood flow into the aortic false lumen ( Fig. 24.1 ). The intent is to mimic the effect of operative repair by isolating the false lumen from the circulation and redirecting all blood flow into the true lumen. As demonstrated in experimental models of dissection, coverage of the primary entry tear is the optimal method of relieving true lumen collapse, restoring perfusion to branch arteries off the true lumen, and promoting thrombosis of the aortic false lumen. Interestingly, dissections with a completely thrombosed aortic false lumen are associated with a favorable prognosis. In contrast, false lumen patency contributes to progressive aortic dilation and is a predictor of late mortality.
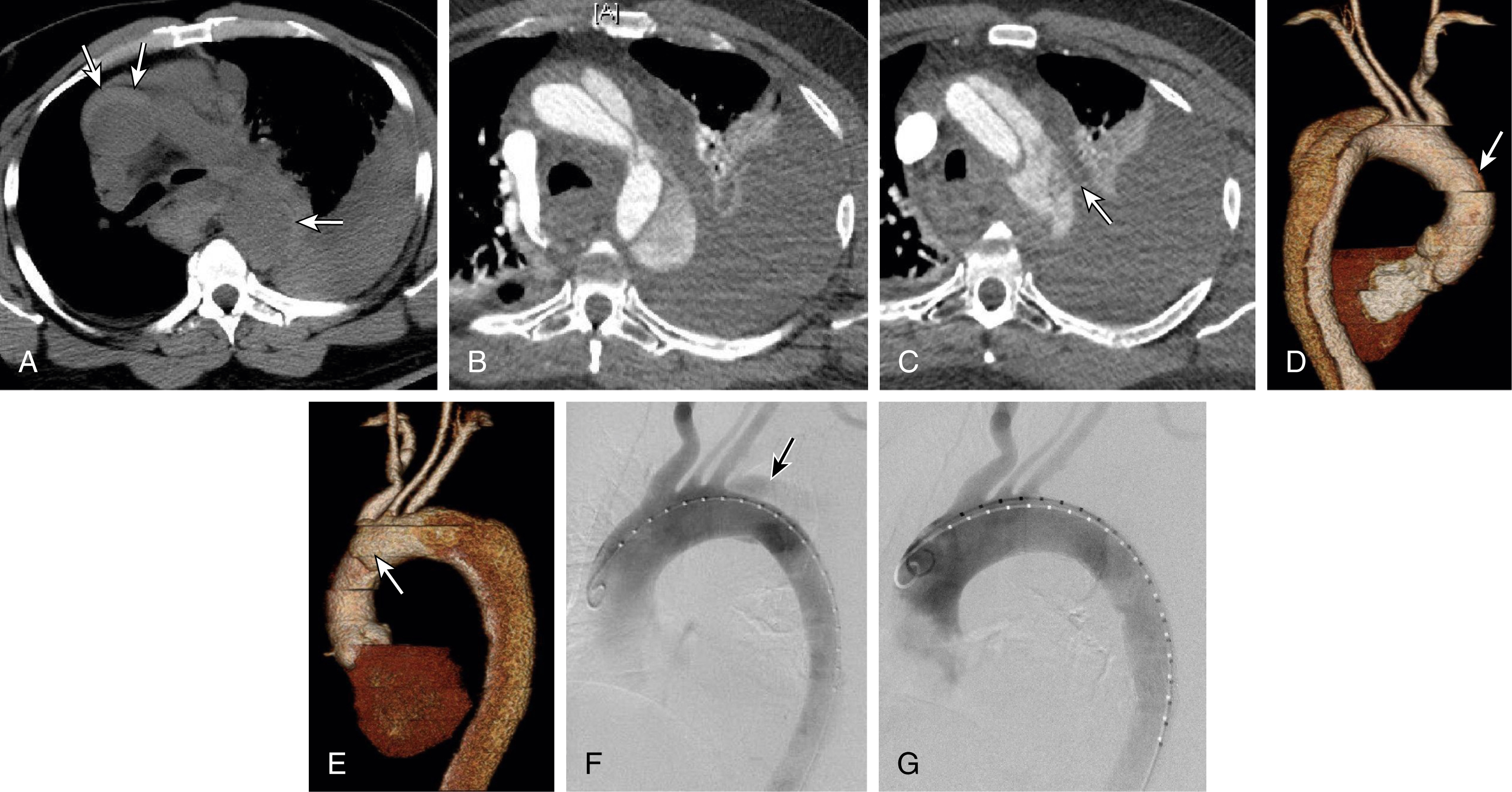
In the typical case of type B dissection, progressive thrombosis of the aortic false lumen after stent-graft placement proceeds distally, irrespective of the location of the primary intimal disruption. The tempo of false lumen thrombosis is variable among individuals and influenced by several factors, such as the size of the false lumen and amount of residual false lumen flow via uncovered additional tears in the septum below the distal margin of the device. Over time the false lumen progressively remodels and shrinks. Although published results of stent-graft management of patients with acute type B dissection are encouraging, it is important to be aware that the literature for aortic dissection often mixes outcomes from a wide variety of clinical contexts in terms of age of dissection, extent of disease, and presence of complications.
Malperfusion syndrome is defined by obstruction of one or more aortic branches, resulting in critical ischemia of the vascular territory supplied by the obstructed branches. Obstruction may result from either a “static” or “dynamic” obstruction. A static obstruction occurs when the dissection directly enters a branch vessel. As the dissection process extends into a branch, it may terminate with false lumen reentry, with a tear in the flap at the distal extent of the false channel, or it may terminate without a reentry tear in the false lumen. The former scenario with branch vessel reentry of the false lumen is more common and results in double-barrel or dual-channel flow to the vascular bed supplied by the involved branch. The other form of static branch vessel involvement with no reentry of the false lumen is less common and is typically associated with tissue malperfusion. Without a reentry tear, there is no flow via the blind sac of the false lumen, which progressively enlarges to its distal extent and may critically obstruct or markedly narrow the true lumen. Thus, the vascular territory supplied by the affected branch is frequently ischemic, and tissue necrosis may occur without expeditious intervention with reperfusion.
Alternatively, dynamic obstruction occurs when the aortic true lumen is collapsed and flow to its branches is compromised by the dissection flap and usually much larger false lumen. Dynamic branch vessel obstruction is frequently associated with a large proximal primary entry tear and a relatively circumferential intimal dissection. The extent of true lumen obliteration is commonly greatest at the levels of the distal descending and proximal abdominal segments. A slitlike aortic true lumen below the diaphragm often courses anteriorly to give origin to the celiac trunk and superior mesenteric arteries. In these cases, the true lumen may be barely perceptible as a wafer-thin crescent, with the dissection septum prolapsed over the origins of true lumen branches like a curtain. The severity of dynamic obstruction may be variable owing to constant motion of the dissection flap, particularly in the acute phase, and to hemodynamic changes in blood pressure and heart rate.
In an individual patient, both static and dynamic mechanisms of branch vessel involvement with or without resultant ischemia may coexist. Branch vessel malperfusion occurs in 30%–50% of all aortic dissections and yields an almost threefold increase in the risk of in-hospital mortality with acute type B dissection. Given the markedly high operative mortality rate with malperfusion, endovascular options are attractive. Malperfusion syndrome can be treated by thoracic aortic stent-grafting and coverage of the primary intimal tear, distal aortic intimal flap fenestration, branch vessel stenting, or a combination of all three.
Surgical intervention for rupture or impending rupture in type B dissections also carries an extremely high operative mortality. Acutely, coverage of the intimal tear with an appropriately sized stent-graft may effectively exclude a rupture or stabilize the dissection while improving true lumen flow. Moreover, exclusion of the primary intimal tear and precluding false lumen patency may reduce some of the late sequelae of type B dissections (aneurysmal dilation, rupture, and mortality) by promoting false lumen thrombosis. The feasibility of successful stent-grafting depends on appropriate selection of patients with suitable aortic anatomy, which ideally includes a sufficient proximal landing zone (usually 15–20 mm) in a relatively disease-free segment of nondissected aorta.
One of the earliest endovascular treatments in the setting of acute type B dissection was performed in 1935 by Gurin, who fenestrated an intimal flap distally to treat lower extremity ischemia. This strategy treats a nonreentry aortic false lumen and dynamic obstruction of the aortic true lumen by decreasing resistance to false lumen outflow rather than treating the proximal tear. Percutaneous balloon fenestration can be used to mitigate the effects of dynamic aortic true lumen obstruction by creating an artificial tear in the dissection septum that enhances communication of flow between true and false lumens.
Similar to Gurin’s original attempt, flap fenestration can be applied for lower extremity ischemia. In this procedure, the intimal flap is punctured and transgressed, usually with a needle/sheath combination using fluoroscopic and/or intravascular ultrasound imaging guidance. In most cases, to facilitate easy targeting, the puncture is performed from the smaller aortic true lumen to the larger false lumen. Once the flap is successfully penetrated, a guidewire and balloon catheter are then advanced to a position that bridges the septum. Commonly, a series of successively larger balloons up to 25 or 30 mm in diameter are inflated to create an adequate fenestration. Limitations include continued false lumen flow, which may expose patients to increased late complications of aortic dissection, particularly aneurysm formation. However, fenestration may provide a treatment option in patients with malperfusion who are poor surgical candidates or who have unsuitable anatomy for stent-grafting.
Finally, uncovered stent placement either within the aorta or (more typically) within compromised aortic branch vessels may further improve flow to an ischemic region. Indications for an uncovered stent include inadequate relief of static obstruction of a branch vessel after open aortic surgery, stent-grafting, or septal fenestration. Uncovered stent placement may also be used alone or as an adjunct to percutaneous balloon fenestration of the dissection septum to increase aortic true lumen diameter.
In a published report, Dake et al. detailed outcomes in patients with acute complicated type B aortic dissection. Eleven presented with symptomatic branch vessel obstruction involving 38 abdominal arterial beds. Of these compromised vessels, 22 were obstructed exclusively by a dynamic process associated with aortic true lumen obliteration, 15 by both dynamic and static mechanisms of branch involvement, and 1 by static obstruction alone. After endograft placement over the primary entry tear, all 22 of the branch vessels obstructed exclusively by a dynamic process and 6 of the 15 arteries with a combination of dynamic and static branch involvement were immediately reperfused. Adjunctive endovascular procedures were used to relieve persistent ischemia in the other obstructed cases.
Dissection variants like intramural hematoma (IMH) and penetrating atherosclerotic ulcer add additional complexity to treatment and indications for intervention for acute aortic disease. Some 16%–36% of IMHs progress to classic aortic dissection. Moreover, because a significant percentage of patients with IMH and no apparent intimal tear on noninvasive imaging actually have a visible tear at the time of operation or autopsy, it is difficult to clearly distinguish IMH and aortic dissection. There is a general consensus that IMH be treated similarly to classic aortic dissection in the corresponding aortic segment. Penetrating atherosclerotic ulcers, which are often associated with IMH and pseudoaneurysm, may be amenable to surgical or endovascular intervention, but affected patients are often very ill and have significant atherosclerotic disease throughout the vascular tree that may lead to difficulty with endovascular access and stent-graft deployment.
It is important to note that acute aortic dissection in patients with Marfan syndrome or other connective tissue disorders should be treated uniquely. Although initial management of the acute dissection follows the same overall principles, there is a general consensus that stent-grafts and other endovascular interventions be used with extreme caution if at all. Not only are these patients often young and the long-term durability of stent-grafts not yet known, there is also significant concern regarding the continuous radial forces on a diseased aortic wall induced by stent-grafts. Further, stent-grafts may promote aortic dissection in some genetic aortopathies. Thus endovascular intervention in patients with connective tissue disorders is not recommended unless there is a clear indication for intervention and the patient presents with prohibitive operative risk factors.
Over the last decade, the scope of interest for understanding dissection has evolved to include studies focused on identifying factors that can predict disease progression in patients with uncomplicated type B dissection managed with medical therapy and in those patients treated with thoracic endovascular aortic repair (TEVAR).
Indeed, many reports have detailed individual prognostic features for disease progression with false lumen enlargement and/or rupture in patients with a diagnosis of initially uncomplicated type B dissection. Both patient demographic factors and anatomic features of the dissection process that influence disease progression have been detailed; however, the list of these characteristics is long and confounding.
Often in an individual patient there are some high-risk criteria identified, but not other high-risk markers. The net result can be confusing and noncontributory when attempting to determine the relative risk of developing complications in a particular patient. Fortunately, more recent reports that have good longitudinal imaging surveillance have been able to effectively boil down the prospective predictors into simple algorithmic formulas capable of separating patients into high-, low-, and intermediate-risk groups.
Similarly, other studies have been looking at defining risk factors for disease progression in patients treated with TEVAR. Perhaps not surprisingly, the considerations are different. It is anticipated that the use of advanced imaging techniques, including 4D magnetic resonance flow studies, will contribute to more accurately tuning the algorithmic factors that can lead to a better stratification of patients into different risk profiles.
Other areas of recent clinical research concentration have included evaluations of the outcomes of TEVAR versus medical therapy for patients with uncomplicated type B dissection and comparisons of the results of TEVAR versus open surgical repair for complicated type B disease.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here