Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Inward elastic recoil of the lung opposes outward elastic recoil of the chest wall, and the balance of these forces determines static lung volumes.
Surface tension within the alveoli contributes significantly to lung recoil and is reduced by the presence of surfactant, although the mechanism by which this occurs is poorly understood.
Compliance is defined as the change in lung volume per unit change in pressure gradient, and may be measured for the lung, the thoracic cage or both.
Various static lung volumes may be measured, and the volumes obtained are affected by a variety of physiological and pathological factors.
An isolated lung will tend to contract until eventually all the contained air is expelled. In contrast, when the thoracic cage is opened it tends to expand to a volume about 1 L greater than functional residual capacity (FRC). Thus in a relaxed subject with an open airway and no air flowing, for example, at the end of expiration or inspiration, the inward elastic recoil of the lungs is exactly balanced by the outward recoil of the thoracic cage.
The movements of the lungs are entirely passive and result from forces external to the lungs. With spontaneous breathing, the external forces are the respiratory muscles, whereas artificial ventilation is usually in response to a pressure gradient developed between the airway and the environment. In each case, the pattern of response by the lung is governed by the physical impedance of the respiratory system. This impedance, or hindrance, has numerous origins, the most important of which are the following:
Elastic resistance of lung tissue and chest wall
Resistance from surface forces at the alveolar gas–liquid interface
Frictional resistance to gas flow through the airways
Frictional resistance from deformation of thoracic tissues (viscoelastic tissue resistance)
Inertia associated with movement of gas and tissue.
The last three may be grouped together as nonelastic resistance or respiratory system resistance; they are discussed in Chapter 3 . They occur while gas is flowing within the airways, and work performed in overcoming this ‘frictional’ resistance is dissipated as heat and lost.
The first two forms of impedance may be grouped together as ‘elastic’ resistance. These are measured when gas is not flowing within the lung. Work performed in overcoming elastic resistance is stored as potential energy, and elastic deformation during inspiration is the usual source of energy for expiration during both spontaneous and artificial breathing.
This chapter is concerned with the elastic resistance afforded by the lungs and chest wall, which will be considered separately and then together. When the respiratory muscles are totally relaxed, these factors govern the resting end-expiratory lung volume or FRC; therefore lung volumes will also be considered in this chapter.
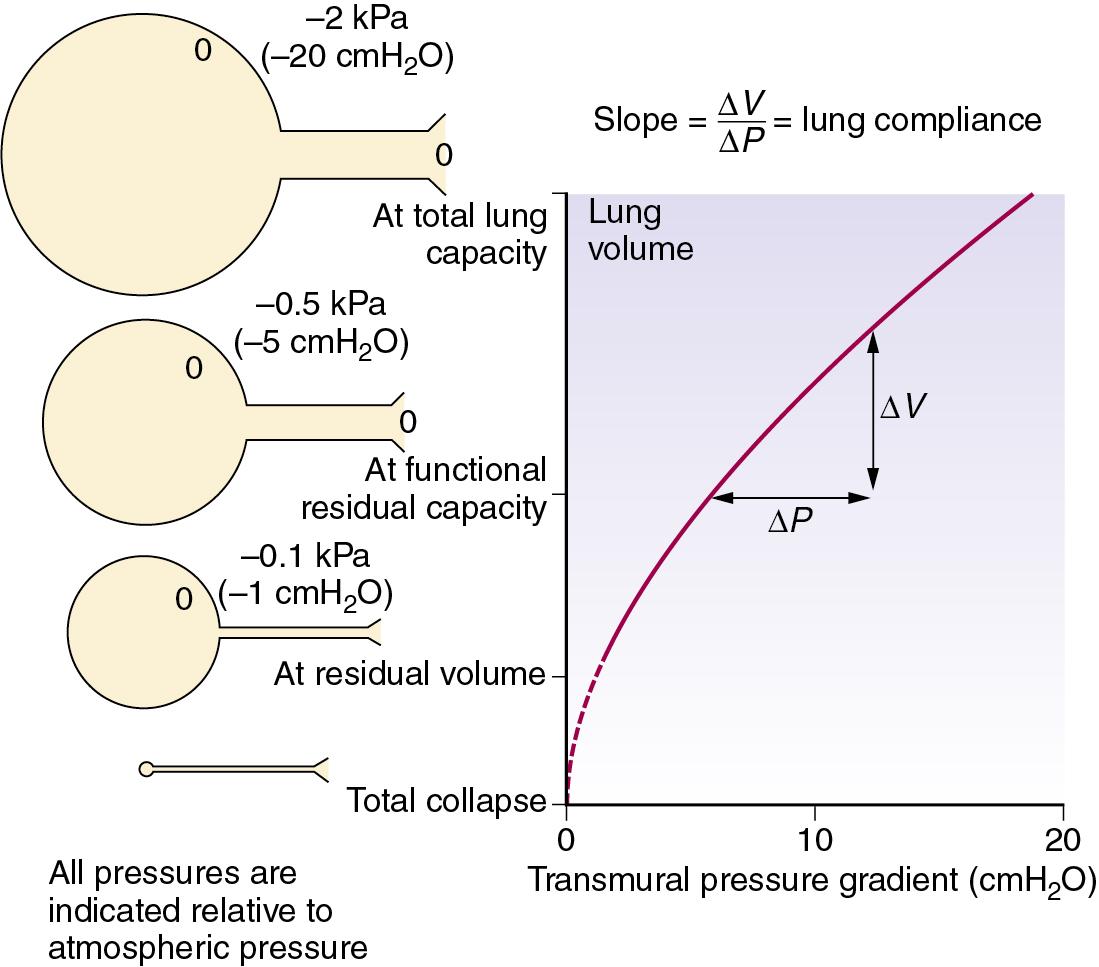
Lung compliance is defined as the change in lung volume per unit change in transmural pressure gradient (i.e., between the alveolus and pleural space). Compliance is usually expressed in litres (or millilitres) per kilopascal (or centimetres of water), with a normal value of 1.5 L.kPa −1 (150 mL.cmH 2 O −1 ). Stiff lungs have a low compliance.
Compliance may be described as static or dynamic, depending on the method of measurement (page 23). Static compliance is measured after the lungs have been held at a fixed volume for as long as is practicable, whereas dynamic compliance is usually measured in the course of normal rhythmic breathing. Elastance is the reciprocal of compliance and is expressed in kilopascals per litre. Stiff lungs have a high elastance.
For many years it was thought that the recoil of the lung was caused entirely by stretching of the yellow elastin fibres present in the lung parenchyma. In 1929 von Neergaard (see section on Lung Mechanics in Chapter 35 ) showed that a lung completely filled with and immersed in water had an elastance that was much less than the normal value obtained when the lung was filled with air. He correctly concluded that much of the ‘elastic recoil’ was caused by surface tension acting throughout the vast air/water interface lining the alveoli.
Surface tension at an air/water interface produces forces that tend to reduce the area of the interface. Thus the gas pressure within a bubble is always higher than the surrounding gas pressure because the surface of the bubble is in a state of tension. Alveoli resemble bubbles in this respect, although the alveolar gas is connected to the exterior by the air passages. The pressure inside a bubble is higher than the surrounding pressure by an amount depending on the surface tension of the liquid and the radius of curvature of the bubble according to the Laplace equation:
where P is the pressure within the bubble (dyn.cm −2 ), T is the surface tension of the liquid (dyn.cm −1 ) and R is the radius of the bubble (cm). In coherent SI units (see Appendix A ), the appropriate units would be pressure in pascals (Pa), surface tension in newtons/metre (N.m −1 ) and radius in metres (m).
Figure 2.1 , A, left, shows a typical alveolus with a radius of 0.1 mm. Assuming that the alveolar lining fluid has a normal surface tension of 20 mN.m −1 (20 dyn.cm −1 ), the pressure within the alveolus will be 0.4 kPa (4 cmH 2 O), which is rather less than the normal transmural pressure at FRC. If the alveolar lining fluid had the same surface tension as water (72 mN.m −1 ), the lungs would be very stiff.
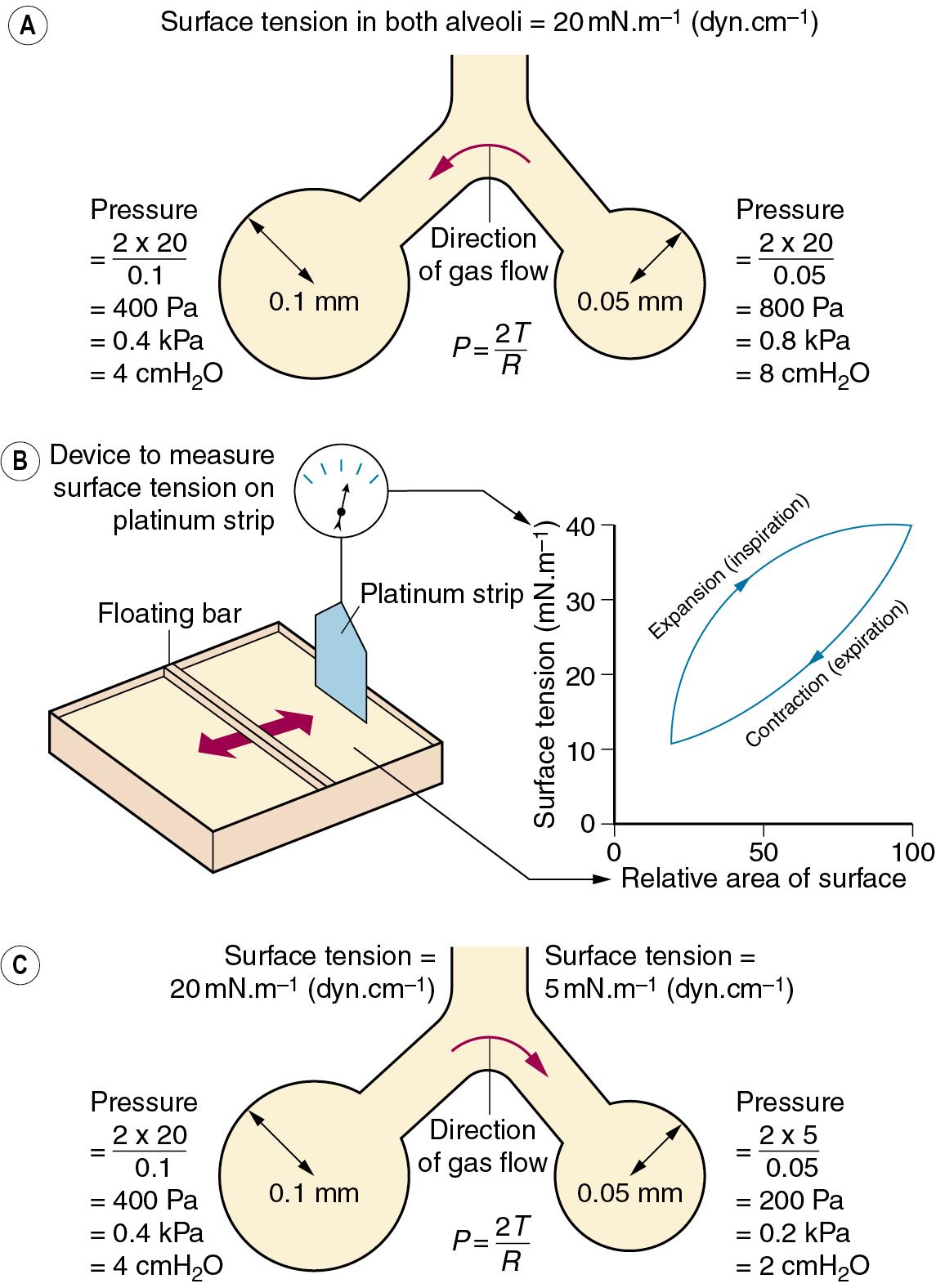
The alveolus in Figure 2.1 , A, right, has a radius of only 0.05 mm, and the Laplace equation indicates that, if the surface tension of the alveolus is the same, its pressure should be double the pressure in the left-hand alveolus. Thus gas would tend to flow from smaller alveoli into larger alveoli and the lung would be unstable which, of course, is not true. Similarly, the retractive forces of the alveolar lining fluid would increase at low lung volumes and decrease at high lung volumes, which is exactly the reverse of what is observed. These paradoxes were clear to von Neergaard, and he concluded that the surface tension of the alveolar lining fluid must be considerably less than would be expected from the properties of simple liquids and, furthermore, that its value must be variable. Observations 30 years later confirmed this when alveolar extracts were shown to have a surface tension much lower than water and which varied in proportion to the area of the interface. Figure 2.1 , B, shows an experiment in which a floating bar is moved in a trough containing an alveolar extract. As the bar is moved to the right, the surface film is concentrated and the surface tension changes, as shown in the graph on the right of the figure. During expansion, the surface tension increases to 40 mN.m −1 , a value which is close to that of plasma but, during contraction, the surface tension falls to 19 mN.m −1 , a lower value than any other body fluid. The course of the relationship between pressure and area is different during expansion and contraction, and a loop is described.
The consequences of these changes are important. In contrast to a bubble of soap solution, the pressure within an alveolus tends to decrease as the radius of curvature is decreased. This is illustrated in Figure 2.1 , C , in which the right-hand alveolus has a smaller diameter and a much lower surface tension than the left-hand alveolus. Gas tends to flow from the larger to the smaller alveolus, and stability is maintained.
The low surface tension of the alveolar lining fluid and its dependence on alveolar radius are because of the presence of a surface-active material known as surfactant. Approximately 90% of surfactant consists of lipids, and the remainder is proteins and small amounts of carbohydrate. Most of the lipid is phospholipid, of which 70% to 80% is dipalmitoyl phosphatidyl choline (DPPC), the main constituent responsible for the effect on surface tension. The fatty acids are hydrophobic and generally straight, lying parallel to each other and projecting into the gas phase. The other end of the molecule is hydrophilic and lies within the alveolar lining fluid. The molecules are thus confined to the surface where, being detergents, they lower surface tension in proportion to the concentration at the interface.
Approximately 2% of surfactant by weight consists of surfactant proteins (SPs), of which there are four types labelled A to D. , SP-B and SP-C are small proteins vital to the stabilization of the surfactant monolayer (see later); a congenital lack of SP-B results in severe and progressive respiratory failure, and genetic abnormalities of SP-C lead to pulmonary fibrosis in later life. SP-A, and to a lesser extent SP-D, are involved in the control of surfactant release, and possibly the prevention of pulmonary infection (see later).
Surfactant is both formed in and liberated from the alveolar epithelial type II cell (page 11). The lamellar bodies (see Fig. 1.11 ) contain stored surfactant that is released into the alveolus by exocytosis in response to high volume lung inflation, increased ventilation rate or endocrine stimulation. After release, surfactant initially forms areas of a lattice structure termed tubular myelin, which is then reorganized into monolayered or multilayered surface films. This conversion into the functionally active form of surfactant is critically dependent on SP-B and SP-C (see later). , The alveolar half-life of surfactant is 15 to 30 h, with most of its components recycled by type II alveolar cells. SP-A is intimately involved in controlling the surfactant present in the alveolus, with type II alveolar cells having SP-A surface receptors, the stimulation of which exerts negative feedback on surfactant secretion and increases reuptake of surfactant components into the cell.
To maintain the stability of alveoli as shown in Figure 2.1 , surfactant must alter the surface tension in the alveoli as their size varies with inspiration and expiration. A simple explanation of how this occurs is that during expiration, as the surface area of the alveolus diminishes, the surfactant molecules are packed more densely, and so exert a greater effect on the surface tension, which then decreases as shown in Figure 2.1 , B . In reality, the situation is more complex, and at present poorly elucidated. The classical explanation, referred to as the ‘squeeze out’ hypothesis, is that as a surfactant monolayer is compressed, the less stable phospholipids are squeezed out of the layer, increasing the amount of stable DPPC molecules which have the greatest effect in reducing surface tension. Surfactant phospholipid is also known to exist in vivo in both monolayer and multilayer forms, and it is possible that in some areas of the alveoli the surfactant layer alternates between these two forms as alveolar size changes during the respiratory cycle. This aspect of surfactant function is entirely dependent on the presence of SP-B, a small hydrophobic protein that can be incorporated into a phospholipid monolayer, and SP-C, a larger protein with a hydrophobic central portion allowing it to span a lipid bilayer. When alveolar size reduces and the surface film is compressed, SP-B molecules may be squeezed out of the lipid layer, changing its surface properties, whereas SP-C may serve to stabilize bilayers of lipid to act as a reservoir from which the surface film reforms when alveolar size increases.
Pulmonary transudation is also affected by surface forces. Surface tension causes the pressure within the alveolar lining fluid to be less than the alveolar pressure. Because the pulmonary capillary pressure in most of the lung is greater than the alveolar pressure (page 340), both factors encourage transudation, a tendency checked by the oncotic pressure of the plasma proteins. Thus the surfactant, by reducing surface forces, diminishes one component of the pressure gradient and helps to prevent transudation.
Surfactant also plays an important part in the immunology of the lung. The lipid component of surfactant has antioxidant activity and may attenuate lung damage from a variety of causes, suppressing some groups of lymphocytes, theoretically protecting the lungs from autoimmune damage. In vitro studies have shown that SP-A or SP-D can bind to a wide range of pulmonary pathogens, including viruses, bacteria, fungi, Pneumocystis jirovecii and Mycobacterium tuberculosis. Polymorphisms of the surfactant genes are associated with the severity of some respiratory diseases; for example, the likelihood of developing severe pulmonary manifestations of an influenza infection is influenced by a single nucleotide polymorphism of the gene encoding SP-B. Acting via specific surface receptors, both SP-A and SP-D activate alveolar neutrophils and macrophages and enhance the phagocytic actions of the latter during lung inflammation.
Treating surfactant-lined alveoli as bubbles that obey Laplace’s law has aided the understanding of lung recoil in health and disease for many decades (see section on Lung Mechanics in online Chapter 35 : The History of Respiratory Physiology). This ‘bubble model’ of alveolar stability is not universally accepted, and there is evidence that the real situation is more complex. Arguments against the bubble model include the following:
In theory, differing surface tensions in adjacent alveoli cannot occur if the liquid lining the alveoli is connected by a continuous liquid layer.
When surfactant layers are compressed at 37°C, multilayered ‘rafts’ of dry surfactant form, although inclusion of surfactant proteins reduces this physicochemical change.
Alveoli are not shaped like perfect spheres with a single entrance point; they are variable polyhedrons with convex bulges in their walls where pulmonary capillaries bulge into them (see Fig. 1.7 ).
Two quite different alternative models have been proposed:
Morphological model. Hills’ model proposes that the surfactant lining alveoli results in a ‘discontinuous’ liquid lining. Based on knowledge of the physical chemistry of surfactants, this model shows that surfactant phospholipids are adsorbed directly onto the epithelial cell surface, forming multilayered rafts of surfactant ( Fig. 2.2 ). These rafts cause patches of the surface to become less wettable, and these areas are interspersed with fluid pools. Surface forces generated by the interaction between the ‘dry’ areas of surfactant and the areas of liquid are theoretically large enough to maintain alveolar stability. The rafts of surfactant may be many layers thick, and are believed to form and disperse with each breath; their function is almost certainly dependent on both SP-B and SP-C.
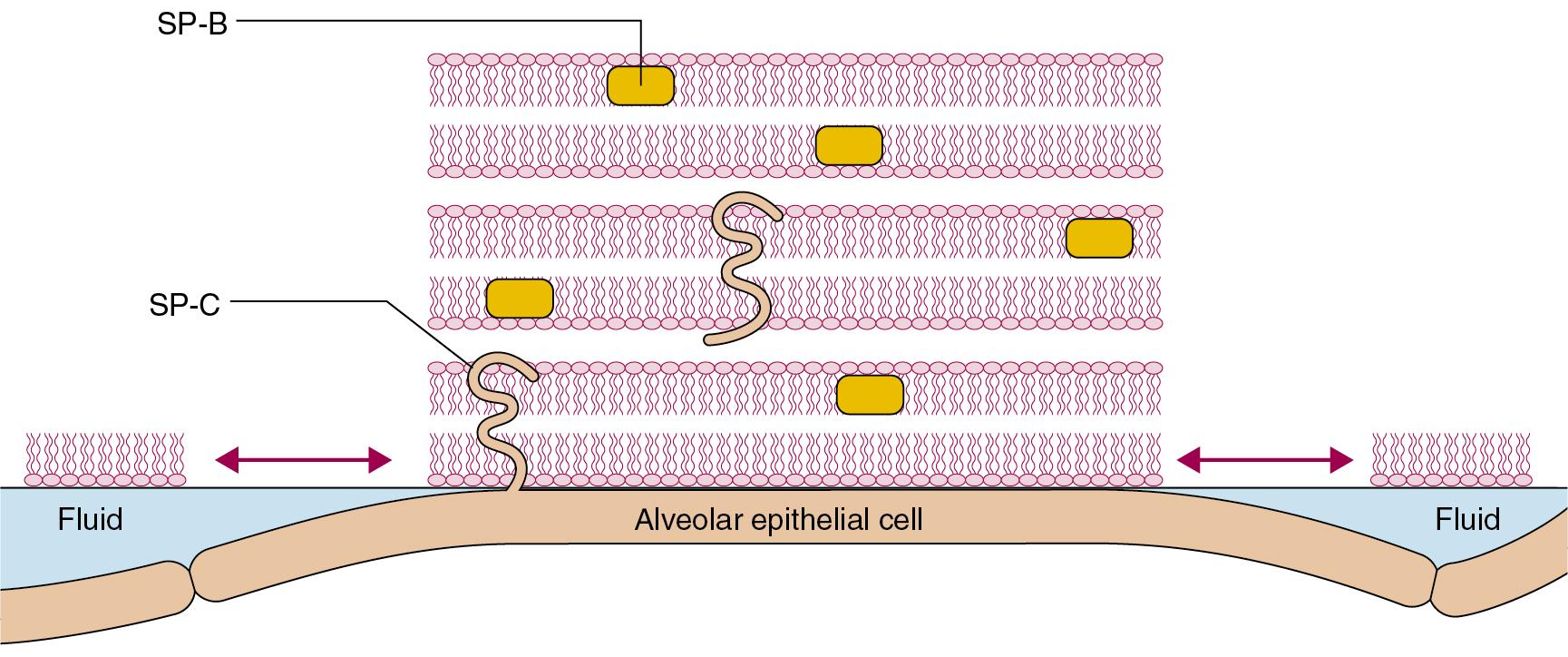
Foam model. Scarpelli developed different techniques for preparing lung tissue for microscopy. By maintaining tissue in a more natural state than previous studies, including keeping lung volume close to normal, he suggested that in vivo there are bubble films across alveolar entrances, across alveolar ducts and within respiratory bronchioles. In this model, each acinus may be considered as a series of interconnected, but closed, bubbles forming a stable ‘foam’. The bubble films are estimated to be thin enough to offer minimal resistance to gas diffusion, the normal mechanism by which gas movement occurs in a single pulmonary acinus (page 7).
More research is clearly needed to either confirm or refute these models.
The transmural pressure gradient is the difference between intrathoracic (or ‘intrapleural’) and alveolar pressure. The pressure within an alveolus is always greater than the pressure in the surrounding interstitial tissue, except when the volume has been reduced to zero. By increasing lung volume, the transmural pressure gradient steadily increases, as shown for the whole lung in Figure 2.3 . If an appreciable pneumothorax is present, the pressure gradient from alveolus to pleural cavity provides a measure of the overall transmural pressure gradient. Otherwise, the oesophageal pressure may be used to indicate the pleural pressure, but there are conceptual and technical difficulties. The technical difficulties are considered at the end of this chapter, whereas some of the conceptual difficulties are indicated in Figure 2.4 .
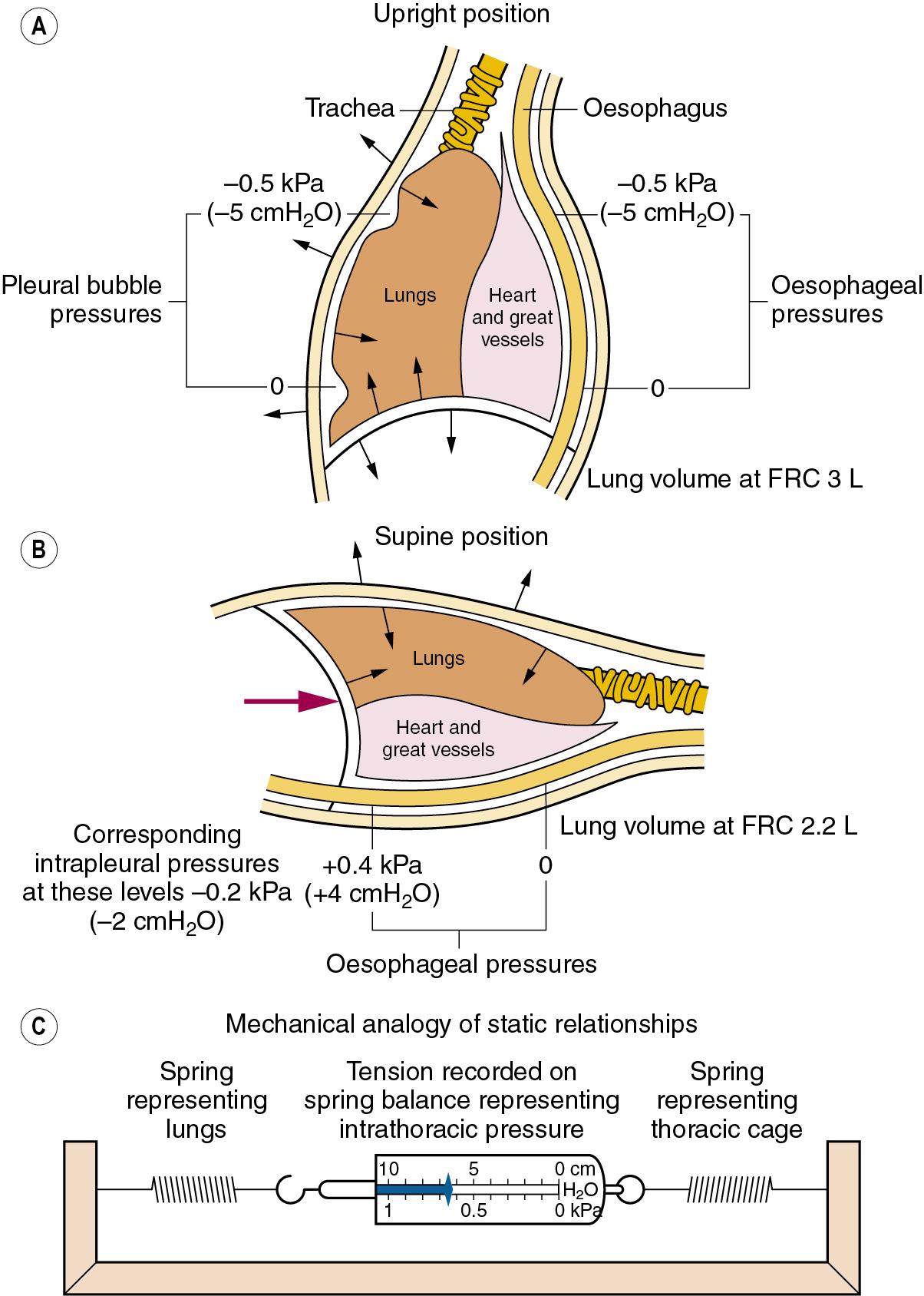
The alveoli in the upper regions of the lung have a larger volume than those in the lower regions, except at total lung capacity. The greater degree of expansion of the alveoli in the upper part results in a greater transmural pressure gradient, which decreases steadily down the lung at approximately 0.1 kPa (or 1 cmH 2 O) per 3 cm of vertical height; such a difference is indicated in Figure 2.4 , A . Because the pleural cavity is normally empty, it is not strictly correct to speak of an intrapleural pressure; furthermore, it would not be constant throughout the pleural ‘cavity’. One should think rather of the relationship shown in Figure 2.3 as applying to various horizontal strata of the lung, each with its own volume and therefore its own transmural pressure gradient on which its own intrapleural pressure would depend. The transmural pressure gradient has an important influence on many aspects of pulmonary function, so its horizontal stratification confers a regional difference on many features of pulmonary function, including airway closure and ventilation/perfusion ratios, and therefore gas exchange. These matters are considered in detail in Chapters 3 and 7 .
At first sight it might be thought that the subatmospheric intrapleural pressure would result in the accumulation of gas evolved from solution in blood and tissues. In fact the total of the partial pressures of gases dissolved in blood, and therefore tissues, is always less than 1 atm (see Table 25.2 ), and this factor keeps the pleural cavity free of gas.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here