Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
As first described by Virchow in 1856, the triad of hypercoagulability, vascular stasis and vascular trauma still remains the critical factors associated with vascular thrombosis. Hypercoagulability causes abnormal thrombosis, which may occur in the venous or arterial circulation, as a consequence of both inherited conditions and/or acquired deficits. Different hypercoagulable states, also known as thrombophilias, may contribute to acute limb, or visceral ischemia, stroke, or cardiac thrombosis. As such, it is critical for vascular surgeons to be well versed with the contemporary diagnosis and management of hypercoagulable disorders.
The pathophysiology and molecular genetics of many thrombophilias have been elucidated over the past 50 years, and most can be managed using evidence-based algorithms. In most circumstances, the hypercoagulable state can be diagnosed by identifying the specific role the deficient factor plays in normal hemostasis. In addition, some individuals may have an inherited defect, but not develop a hypercoagulable state until they acquire an additional risk factor. This chapter provides a logical approach to the diagnosis and management of different hypercoagulable states.
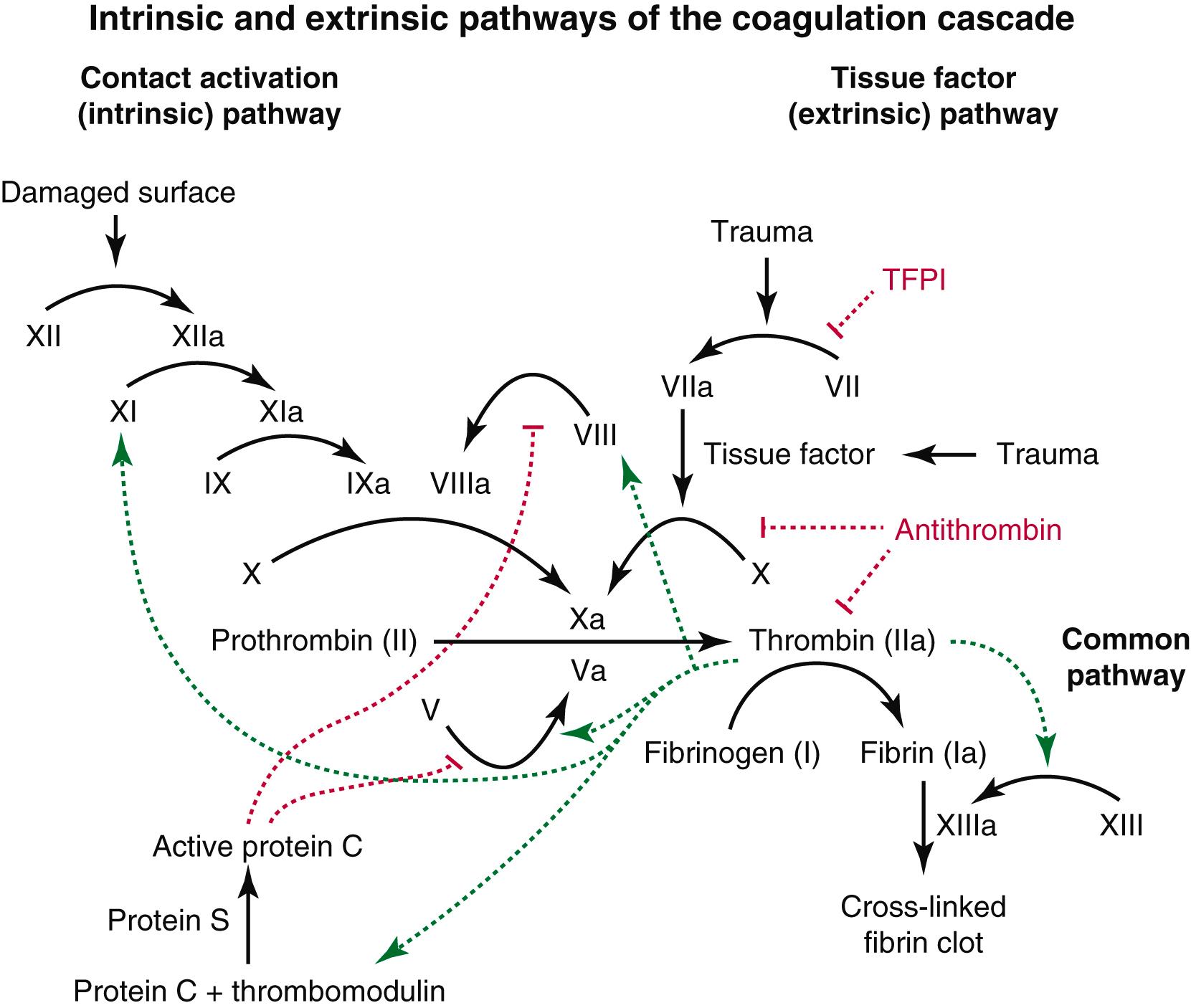
Coagulation is an inherent property of the hematologic system and blood vessel wall. Under normal conditions, the fluidity of blood flow is maintained by the balance between the pro-coagulant and antithrombotic factors contained within the blood and on the endothelial surface of the vessel wall. When endothelial injury occurs, primary hemostasis involves focal vasoconstriction from release on endothelin and secretion of von Willebrand factor, which initiates platelet adhesion and activation. Activated platelets secrete granules such as adenosine diphosphate, serotonin, and thromboxane A2, which in turn induces other platelets to congregate and form a platelet plug. If the vascular injury is small, the platelet plug may be able to form within several seconds and seal the injured area. But when a simple platelet plug is not enough to stop the bleeding, intrinsic and extrinsic pathways of the coagulation cascade are activated to help with clotting within a common pathway ( Fig. 40.1 ). Secondary hemostasis is initiated from release of tissue factor, followed by activation of the coagulation proteins in a series of controlled enzymatic reactions that results in the generation of thrombin and cross-linked insoluble fibrin clot. However, there are also antithrombotic processes designed to counter the over-production of fibrin, involving the release of tissue plasminogen activator and thrombomodulin from the endothelium, and activation of antithrombin, and protein C and S (see Ch. 38 , Normal Coagulation).
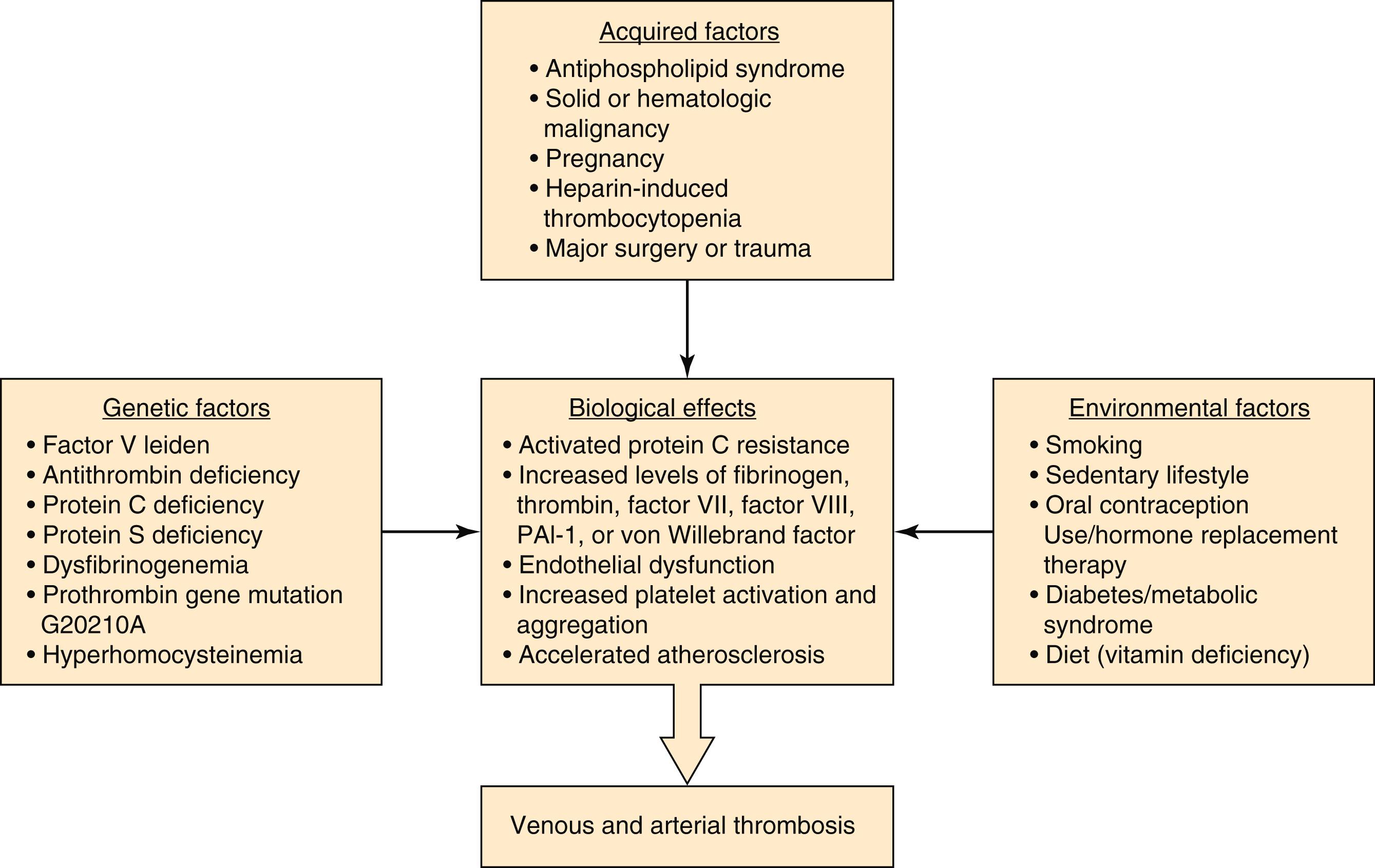
A hypercoagulable state may result from either overactivity of these endogenous procoagulant factors or a deficiency of the antithrombotic factors. This may result from a combination of genetic factors that are inherited and/or numerous environmental factors that an individual may acquire during their lifetime ( Fig. 40.2 ). The interplay of genetic and environmental factors can result in biological effects that increase the risk for thrombosis. Further, hypercoagulability may also result from risk factors that primarily affect the vessel wall. This includes factors such as atherosclerosis, vasculitis, infection, or trauma that can contribute to endothelial disruption and activation of the coagulation cascade. As such, a patient’s lifetime risk for thrombosis can increase by a factor more than the sum of each individual risk factor.
Congenital or hereditary hypercoagulability results from gene mutations in coagulation factors and can be divided into two broad groups using a simple classification system proposed by Crowther and Kelton in 2003 ( Table 40.1 ). Within this system, the first classification group is defined by defects that lead to reduced levels of the natural inhibitors of the coagulation cascade such as antithrombin, protein C, or protein S. These “loss of natural thrombosis inhibition” deficiencies are associated with a high risk of thrombosis during a patient’s lifetime. The second classification group is associated with defects that result in a “gain in procoagulant function” due to increased levels or function of coagulation factors, including factor V Leiden or prothrombin G20210 mutations, increased levels of factor VIII, and the dysfibrinogenemias.
| Inherited Thrombophilia | Prevalence in General Population (%) | Frequency in Patients with VTE (%) | Relative Risk of Initial VTE | Relative Risk of Recurrent VTE |
|---|---|---|---|---|
| Group 1 – Conditions That Lead to Loss of Natural Thrombosis Inhibition | ||||
| Antithrombin III deficiency | 0.02 | 4–7.5 | 10–30 | 3 |
| Protein C deficiency | 0.2–0.5 | 2.5–6 | 10 | 2 |
| Protein S deficiency | 0.1–0.7 | 1.3–5 | 10 | 1 |
| Group 2 – Conditions That Lead to Gain in Procoagulant Function | ||||
| Factor V Leiden heterozygote a | 2–7 | 10–19 | 3–6 | 1.1–1.8 |
| Factor V Leiden homozygote a | 0.06–0.25 | 1.5 | 7–20 | 2–3 |
| Prothrombin G20210A heterozygote a | 1–2 | 5–10 | 3–4 | 0.7–2.3 |
| Prothrombin G20210A homozygote a | Rare | Unknown | 2–20 | Unknown |
| Elevated factor VIII | 11 | 25 | 4.8 b | 10–45 b |
| Hyperhomocysteinemia | 5–10 | 10 | 2–4 | 1 |
| Dysfibrinogenemia | Unknown | <1 | Unknown | Unknown |
a Heterozygous and homozygous carriers of the factor V Leiden and prothrombin G20210A mutations are predominantly found in patients of European descent.
Antithrombin III (AT3) is the most important inhibitor of thrombin and other activated clotting factors, including factors Xa, IXa, and VIIa. The physiologic activity of AT3 is enhanced 1000-fold by the binding of naturally occurring or administered heparin or heparin sulfates. The incidence of inherited AT3 deficiency has been estimated at 1:2000 of the general population ( Table 40.1 ). Among individuals diagnosed with venous thromboembolism (VTE), however, AT3 deficiency has been reported to occur in between 4% to 7.5% of patients. , AT3 is inherited as an autosomal dominant trait, and more than 250 mutations have been described to date. Nearly all patients are heterozygous for AT3 mutations, given that the severity of thrombophilia associated with homozygosity is rarely compatible with life.
AT3 deficiency has been classified into two general subtypes based on how each gene mutation specifically affect antithrombin antigen levels. Type I is characterized by point mutations or gene deletions that lead to low antithrombin antigen and activity levels. In comparison, Type II mutations result from a mutation in the active inhibitory site on the protein that lowers functional antithrombin activity, but does not reduce its levels.
Patients with AT3 deficiency are at a significantly higher risk of venous thrombosis than patients with other hereditary thrombophilias. Approximately 60% of patients with AT3 deficiency will have a VTE event by the age of 60 years, and a family history of thrombosis is usually present. The relative risk of VTE in patients with antithrombin deficiency has ranged between 10- to 30-fold within several large multicenter studies. , As such, screening of first-degree family members is recommended after an AT3 deficiency diagnosis is made in an individual with VTE.
Protein C is a vitamin K-dependent anticoagulation protein that is activated by thrombin to activated protein C (APC). When thrombin levels are high, thrombin binds to the endothelial protein receptor, thrombomodulin (TM), which changes the specificity of thrombin from cleaving fibrinogen or activating platelets to activating protein C. Protein C binds to its specific endothelial receptor, termed the endothelial protein C receptor, which enhances its activation. Mutations that result in deficiency of protein C are found in 0.2%–0.5% of the general population, and in 2.5%–6% of patients with VTE ( Table 40.1 ). , ,
Hundreds of mutations resulting in protein C deficiency have been reported to date. The majority of people with protein C deficiency lack one copy of the functioning genes leading to activity levels less than 60%, whereas homozygous mutations are rare. These mutations have been classified into two general subtypes: type I mutations are considered quantitative defects and have reduced functional and antigenic protein levels of protein C. In comparison, type II mutations have reduced functional levels but preserved antigen levels of the protein. These are considered qualitative mutations that prevent protein C from interacting with other molecules such as thrombomodulin, phospholipids, and factors V or VIII.
Patients heterozygous for protein C mutations typically present with VTE involving the lower extremities. In comparison, homozygosity for protein C deficiency may result in nearly absent protein C activity and present at birth as a neonatal disorder termed purpura fulminans. This disorder is characterized by diffuse microvascular thrombosis of the skin and systemic organs, and immediate treatment with heparin, plasma, or protein C concentrates are required to prevent neonatal death. The majority of homozygous neonates with protein C deficiency will have functional levels less than 20% of normal.
Protein S is the vitamin K-dependent cofactor necessary for the inactivation of factors Va and VIIIa by APC. Protein S exists in two forms: the functionally active free form that usually constitutes 20% to 40% of the total protein, and the remaining 60% to 80% that is active and bound to complement binding protein C4b. , Protein S deficiency can be found in up to 0.7% of the population and result from mutations that cause low levels of synthesis, increased proteolytic cleavage, and/or increased binding to C4b. Most patients with inherited protein S deficiency will have activity levels between 50% and 75% of normal.
Protein S mutations can be classified into three different subtypes. Type I mutations are characterized by reduced functional and antigen protein levels of protein S. Type II has reduced functional activity of protein S, but normal antigen levels. And type III has normal antigen levels, but reduced free active protein S due to enhanced C4b binding. Type I and type III protein S deficiencies are the most common forms of the deficiency encountered.
A deficiency in protein S is phenotypically similar to protein C deficiency and patients typically present with VTE. However, protein S levels can also drop during the second and third trimesters of pregnancy, and woman may have fetal loss as their only manifestation of this disorder. Reduced protein S levels have also been reported in patients with acquired conditions associated with thrombosis, which includes active cancer, lupus, antiphospholipid antibody syndrome, sepsis, inflammatory bowel disease, and advanced HIV disease. ,
Factor V is a cofactor that accelerates the conversion of factor II (prothrombin) to thrombin by factor Xa. Under normal circumstances, factor V is degraded by the potent serine protease, activated protein C (APC). APC cleaves the protein at two sites, which helps modulate thrombin generation and subsequent clot formation. The most common mutation in factor V is a point mutation in the 506 position that results in a substitution of glycine for arginine ( aka factor V Leiden). , This mutation renders one of the factor V cleavage sites resistant to the action of APC, and results in a slowing of the inactivation of the cofactor and increased thrombin generation. The factor V Leiden mutation is the most common inherited thrombophilia, occurring in approximately 2% to 7% of individuals of European ancestry. It can be found in up to 19% of people with VTE and in 30% to 50% of individuals being evaluated for thrombophilia ( Table 40.1 ). By comparison, the mutation is very rare in patients of Asian, African American, and Native American descent. Patients heterozygous for the factor V Leiden have a relative risk of thrombosis estimated at 3- to 6-fold, and a cross-sectional study from Italy found that only 6% of heterozygotes developed a thromboembolic event by the age of 65 years. , , Homozygous carriers of this mutation have up to a 20-fold increased relative risk of VTE.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here