Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Vascular physiology is grounded in the following basic equation: CO (flow) equals the pressure difference (Δ P ) divided by the resistance ( R ). Resultantly, coronary blood flow (CBF) is largely driven by supply/demand mismatches in myocardial oxygenation, requiring increased delivery of substrate to the myocardium.
The coronary microcirculation is the primary regulator of myocardial perfusion and is largely dependent on vessel tone in response to endothelial-derived factors such as nitric oxide (NO).
At baseline, the myocardium is almost at maximal oxygen extraction from the circulation. Thus any increase in myocardial oxygen consumption is highly dependent on an increase in CBF.
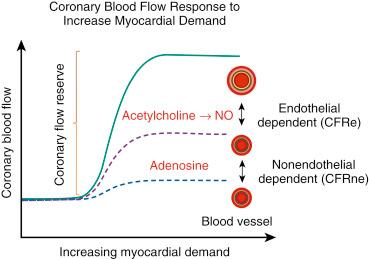
Coronary flow reserve (CFR) is an important concept in coronary physiology as it represents the normal circulatory ability to increase CBF in response to increased demands and stressors on the myocardium.
CFR can be assessed invasively using a Doppler flow wire that captures changes in red cell velocity after the infusion of adenosine; thereafter a ratio of the Doppler velocity to the resting velocity can be obtained.
Abnormal CFR should prompt a workup for underlying diseases that might primarily impair the microcirculation of the myocardium.
Conventional angiography visually and subjectively assesses only approximately 5% to 10% of coronary arteriolar anatomy. The remaining 90% to 95% of the coronary vasculature requires a detailed and systematic approach to successfully evaluate patients with typical symptoms of cardiac angina without obvious obstructive coronary artery disease (CAD). In the visible conduits, an atherosclerotic lesion greater than 70% has traditionally been treated with revascularization—surgical or percutaneous—for the resolution of symptoms. In cases where there is discordance between the patient’s anginal symptoms, noninvasive stress testing, and the coronary luminogram, one must methodically evaluate for coronary microvascular dysfunction (CMD) as a physiologically plausible cause of cardiac chest pain. This chapter aims to inform the reader of the basics of coronary arterial physiology with special attention to the coronary microcirculation, which should be of benefit to the practicing interventional cardiologist.
Current estimates state that anywhere from 40% to 60% of patients undergoing coronary angiography for compelling symptoms or positive noninvasive testing have nonobstructive CAD (NOCAD). Moreover, the majority of patients who have been referred for coronary angiography were found to have microvascular dysfunction. These diagnostic and treatment dilemmas are typically overlooked because percutaneous intervention cannot address the pathophysiologic process. The evaluation of coronary epicardial and microvascular disease on top of traditional risk models like the Framingham score can correctly reclassify cardiovascular risk in nearly one-fourth of patients. That these patients deserve particular attention and further evaluation is underscored by their increased morbidity, mortality, low quality of life, and high medical expenses.
The initial workup of NOCAD should include a good history and physical examination. Some clues in the history—although not absolutely indicative of microvascular disease—could include conventional risk factors (e.g., sex, smoking, hypertension, hyperlipidemia, autoimmune or inflammatory diseases such as hypothyroidism, and Reynaud disease). Other important information to be obtained in the history should include chronic and acute medications and toxin ingestion. Agents documented to cause coronary vasospasm are listed in Table 38.1 .
| Tobacco smoking | Butane |
| Khat | Alcohol |
| Cocaine | Ecstasy/lysergic acid diethylamide |
| Methamphetamines | Triptans/sumatriptans |
| Marijuana | Chemotherapy (5-flououracil, capecitabine) |
Specifically in women, a history of polycystic ovaries and toxemia of pregnancy, and in men the presence of endothelial dysfunction outside of the coronary vasculature, can be associated with CMD. Physical examination is less revealing in CMD. Laboratory abnormalities are typically nonspecific; however, abnormal thyroid function, uric acid levels, markers of inflammation such as lipoprotein PA 2 (LpPA 2 ) and C-reactive protein (CRP), white blood cell counts, and even brain natriuretic peptides can be seen in these patients. Other noninvasive imaging such as coronary computed tomography (CT) can often rule out obstructive CAD; however there is no functional testing component to assess the endothelium or microcirculation. Therefore the utilization of coronary CT may not provide appropriate information on microvascular function.
If clinically compelled by a history of typical cardiac angina, an abnormal noninvasive stress test, or even elevated cardiac biomarkers, cardiac catheterization is performed in these patients, generally revealing no obvious obstructive coronary lesion. One must make sure that obstructive coronary disease is ruled out by thoroughly inspecting the results of angiography to determine whether causative “flush” coronary occlusions or other coronary anomalies may have been missed. In several instances, the presence of coronary vasospasm can be documented; one should use intracoronary nitroglycerin to reduce vascular tone, as this can cause typical angina without obstructive CAD. Furthermore, lesions thought to be nonobstructive in orthogonal views should be carefully evaluated with physiologic means such as fractional flow reserve (FFR) or instantaneous flow reserve (iFR) to make sure that no hemodynamically significant lesion is present. Established metrics for nonobstructive disease in patients with 40% to 70% luminal stenosis include an FFR value above 0.80, or iFR above 0.89. Imaging with intravascular ultrasound (IVUS) or optical coherence tomography (OCT) can also be of importance to investigate potentially unstable/vulnerable plaques that may be smoldering culprit lesions. OCT is also of great importance in patients with chest pain and CMD to rule out spontaneous coronary artery dissection (SCAD), as treatment pathways will certainly diverge based on the underlying diagnosis. Evidence of moderate CAD and conditions such as anemia, tachycardia, sepsis, and so on can cause a type 2 myocardial infarction and should prompt a review looking for the underlying cause of the myocardial supply/demand mismatch, amelioration of the inciting cause, and potential adjustment of secondary prevention medications such as chronotropic, vasodilator, or antiplatelet agents in treating these patients.
Indications for further invasive testing beyond coronary angiography include a discrepancy between the degree of epicardial disease as detected by the coronary angiogram and the patient’s symptoms and/or the results of the noninvasive stress test. Angina or “chest pain syndrome“ can include typical and atypical features, such as exertional dyspnea; chest, neck, and shoulder discomfort; or similar symptoms with certain triggers such as physical activity, anxiety, social situations, or emotional prompts. These patients could also have inconclusive or likely abnormal noninvasive testing such as exercise electrocardiography (ECG), stress imaging, CT, or magnetic resonance imaging (MRI). Stress testing often reproduces symptoms, but there could be some other abnormal component such as poor exertional effort, cardiac output limitation on VO 2 testing, abnormal anaerobic thresholds, abnormal O 2 pulse rise, a sudden increase in heart rate upon reaching anaerobic threshold, or other anginal symptoms. In many cases, noninvasive stress testing can reproduce symptoms in these patients; however, there may be a discrepancy between noninvasive stress testing and both the epicardial and microvascular abnormalities found via invasive angiography.
CT has recently become an emerging diagnostic modality for assessing obstructive coronary disease in low-risk patients. Coronary CT can detect obstructive CAD, and these findings have correlated with overall cardiac outcomes. There are, however, inherent limitations regarding lower sensitivity in women, poor specificity in the setting of coronary calcium, and exposure to radiation and contrast dye. CT-FFR has now developed into a means by which to assess the physiologic significance of obstructive CAD with an improvement in diagnostic accuracy of 36% compared with coronary CT alone (A Randomised Controlled Trial to Compare Routine Pressure Wire Assessment With Conventional Angiography in the Management of Patients With Coronary Artery Disease [RIPCORD]) as well as improved sensitivity and specificity (area under the curve increased to .92) in the Diagnosis of Ischemia-Causing Stenoses Obtained Via Noninvasive Fractional Flow Reserve (DISCOVER-FLOW) trial. CT-FFR values below .80 nearly doubled the probability of revascularization (2.9 to 4.3, P = .03) compared to those above .80 without any change in cardiovascular outcomes in the PROspective Multicenter Imaging Study for Evaluation of Chest Pain (PROMISE) trial with the caveat that only one-third of the patients had diagnostic CT-FFR images. Finally, in results from the Prospective LongitudinAl Trial of FFRct: Outcome and Resource IMpacts (PLATFORM) trial, CT-FFR demonstrated improved specificity in which patients were sent to the catheterization lab without a difference in clinical outcomes. These imaging modalities are certainly promising in the realm of assessing obstructive CAD; however, they have not been tested or validated in assessing the microcirculation—only in excluding obstructive CAD. Further invasive assessments in the catheterization laboratory are likely still necessary should coronary CT be unrevealing.
The primary role of epicardial coronary vessels is conductance, while the microcirculation matches blood supply to myocardial oxygen requirements (mostly though modulation of arteriolar tone) and makes possible the interchange of oxygen, nutrients, and metabolites between myocytes and blood in the capillary network. The mechanism for ischemia generation in epicardial vessels is the impairment of conductance caused by inward plaque growth during atherogenesis, intraluminal obstruction caused by thrombus, and/or coronary spasm. Conversely, CMD results mostly from inadequate coronary arteriolar autoregulation and, less frequently, from structural remodeling of arterioles of the capillary bed, intraluminal plugging, or microvascular edema/hemorrhage impairing the conductance of the microvasculature ( Fig. 38.1 ).
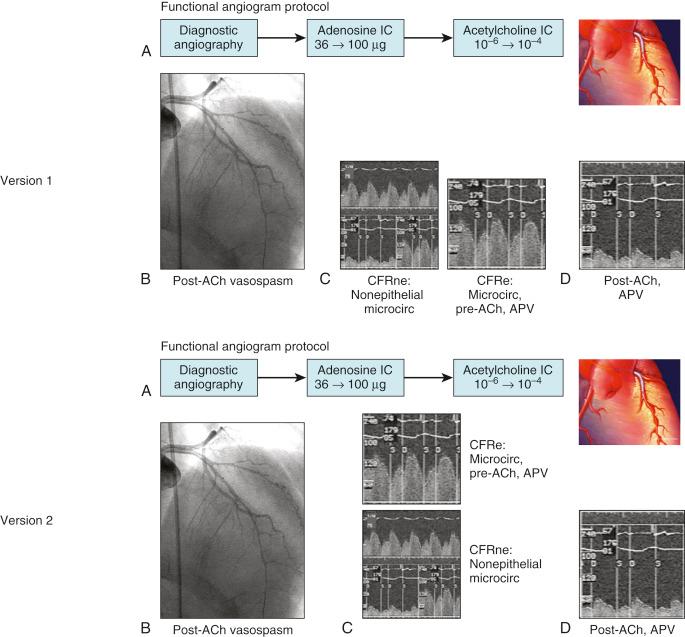
The invasive assessment of vascular function has been historically described and should be divided based on the mechanism of reduction in coronary flow reserve (CFR) to determine if the abnormalities in the CFR are nonendothelial-dependent (CFRne) or endothelial-dependent (CFRe). These invasive measures recorded in the cardiac catheterization lab can be performed with very high fidelity and safety, including intracoronary vascular reactivity testing. Assessing the ability of the microvasculature to meet myocardial demand, CFR is one measure of the ability of the coronary circulation to augment blood flow with stress measured as a ratio of maximal coronary blood flow (CBF) (usually drug-induced) and baseline physiological blood flow.
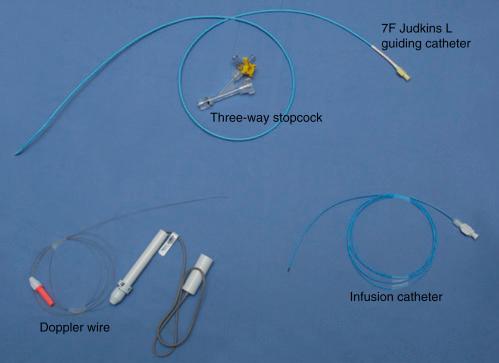
In patients without obstructive CAD as determined by imaging and physiologic assessment wherein further invasive assessment of the microcirculation is indicated, one must carefully plan the procedure, including the equipment used ( Fig. 38.2 ), vessels studied, and order of microvascular assessments. Traditionally these investigations are performed from a femoral approach. This is considered best for two main reasons. First, to avoid the need for vasodilators routinely administered during radial access, and, second, to accommodate the guiding and infusion catheters plus the wire required to perform the test. Ideally all vasoactive drugs should be withheld prior to testing. This is particularly important with regard to nitrates and calcium channel blockers. Other vasoactive substances, such as nicotine and caffeine, should be withheld for 12 hours prior to testing. Furthermore, operators experienced in performing and interpreting these tests should be consulted in the study of these patients so that technical success is maximized in addition to arriving at a proper diagnosis.
Unless otherwise indicated by regional abnormalities on a noninvasive stress testing, the left anterior descending (LAD) artery is the vessel of choice for these studies. This vessel subtends the most myocardium, is typically very approachable with the equipment due to its large caliber, and can easily be assessed in terms of Doppler velocities and diameter quantifications. The left circumflex or right coronary arteries can be studied if inferior or lateral changes are seen on the noninvasive test or if transient ischemic mitral regurgitation secondary to posterior/lateral ischemia is in question. If these arteries are to be studied, changes in the selection of a guiding catheter may have to be made, in addition to reductions in dosing of provocative medications.
Some patients who have previously undergone stent implantation for obstructive CAD can have further angina symptoms without the reemergence of obstructive CAD or restenosis. There are data that show abnormal coronary reactivity in vessel segments distal to the stented segment. Assessing coronary reactivity mainly distal to the stent and pertaining to the microcirculation can be important in evaluating chest pain in these patients. The protocols described below can be conducted distal to previously placed stents using careful wiring of these vessels to avoid trapping the wire beneath a stent strut or deforming the stent.
A 7-Fr guide is predominantly used. This is typically an extra backup (EBU/XB) or Judkins left shape depending on the anatomy ( Figs. 38.3 and 38.4 ). Recently the ability to utilize a 7.5-Fr sheathless guide or 7-Fr radial sheaths and guides has allowed these procedures to be performed using the radial approach. One caveat to this approach is the potential for radial artery spasm requiring intraarterial vasodilators such as nitroglycerin and verapamil, which may influence the results of the catheterization and velocity measurements, thereby interfering with an accurate assessment of the microvasculature. To avoid catheter or wire thrombosis, intravenous heparin should be administered at a standard dose (60 to 80 IU heparin/kg body weight) to ensure an activated clotting time (ACT) greater than 250 seconds. An additional safety measure is having a syringe for intraarterial nitroglycerin readily available should coronary spasm unexpectedly occur.
Once the 7-Fr guide has been positioned within the ostium of the left main coronary artery, the Doppler wire and infusion catheter are positioned. The infusion catheter should be positioned at the proximal to middle portion of the vessel (if the vessel is large enough to accommodate it without creating ischemia) to ensure adequate drug delivery to the distal portion of the artery with plenty of upstream vessel not directly affected by the drug infusion to test upstream flow-mediated vasodilation. Once the vessel has been successfully instrumented with the infusion catheter, one can carefully position the 0.014-inch Doppler velocity wire through the infusion catheter into the vessel being studied. Once testing is ready to begin, the guide and infusion catheter should both be properly flushed with heparinized saline and equalized. The system should not be pretreated with intracoronary nitroglycerin as this will alter blood flow independent of the endothelium or microcirculation with the smooth muscle–directed vasodilation.
An alternative to an infusion catheter placed in the coronary artery is to advance the flow wire into the LAD without the use of an infusion catheter and to infuse drug directly through the guide. While it is overall safe to perform, there is a risk of left main or proximal LAD dissection with this method, and the possibility of vasospasm throughout the left-sided coronary arteries without placement of workable coronary wires in the distal vessels.
It is recommended to begin by obtaining CFRne once the Doppler wire and infusion catheter are both in place. The administration of intracoronary adenosine (up to 72 μg) allows for the measurement of maximal coronary endothelial independent vasodilation (intravenous adenosine would be inexact to test coronary microcirculation). This hyperemic average peak velocity (APV) is compared with the resting APV and a CFRne ratio is obtained (see Fig. 38.1 ). This should be repeated two to three times until a stable maximal APV is obtained.
Once the assessment of the nonendothelial-dependent microcirculation has been ascertained via adenosine administration and the CFRne obtained, the next step in the workup of patients with CMD is assessing the endothelial-dependent epicardial diameter and microcirculation, CFRe (based on changes of APV). This is performed with the intracoronary administration of the endothelium-dependent vasodilator acetylcholine (ACh). This will assess the endothelial-dependent vasodilatory properties of the epicardial, as well as the coronary, microvasculature. Two measurements are needed to calculate CFRe—the APV and the coronary artery luminal diameter.
Following stable positioning of the infusion catheter and with the Doppler wire in place, graded infusion of ACh should be initiated with increasing doses of ACh (10 −6 M → 10 −4 M, or equivalently, ACh 0.001 mmol → 0.1 mmol) for up to 3 minutes at 1 mL/min via an infusion pump. The infusion should be quickly stopped should symptoms or complications occur. During the infusion, concomitant measurement of epicardial diameter via any angiographic imaging software and APV will allow calculation of CBF. The purpose of this testing is twofold: (1) assessment of coronary epicardial vasoreactivity and (2) assessment of the ability of the endothelium of the coronary microcirculation to appropriately increase CBF. This particular protocol involves the measurement of coronary epicardial diameter at three sites along the artery: 5 mm proximal and 5 mm distal to the infusion catheter for CBF calculations (usually mid-LAD), and at the distal LAD , as well as measurement of APV after 3 minutes of each ACh infusion. Hemodynamic data as well as patient symptoms should be assessed at baseline, at the end of each 3-minute period of infusion, and after the administration of 100 to 200 μg of intracoronary nitroglycerin. Some operators will choose to administer ACh, then nitroglycerin, and finish with adenosine to obtain CFRne with the thought being to remove epicardial vessel tone with nitroglycerin prior to adenosine. Both protocols have been used and reported with comparable results.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here