Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Cocaine is an alkaloid derived from the plant Erythroxylon coca and other Erythroxylon species in South America. The leaves contain cocaine as the principal alkaloid, plus a variety of minor alkaloids. Only decocainized coca products are legal in the USA, but some commercially available tea products have been found in the past to contain cocaine in a concentration normally found in coca leaves (about 5 mg of cocaine per 1 g tea-bag). This results in only mild symptoms when package directions to drink a few cups per day are followed, but massive overdosing can result in severe agitation, tachycardia, sweating, and raised blood pressure.
Cocaine, as the leaf or as an extracted substance in different forms, has been used for different purposes. Andean Indians have long chewed leaves of the coca plant to reduce hunger and increase stamina. Pure cocaine was first extracted from coca in the 19th century; it was used to treat exhaustion, depression, and morphine addiction and was available in many patent medicines, tonics, and soft drinks. In the USA, after the Harrison Narcotic Act of 1914 and the Narcotic Drugs Import and Export Act of 1922, the use of cocaine fell, and the National Commission on Marihuana and Drug Abuse in 1973 reported that cocaine was little used. Since then, however, use has grown and there is now an epidemic in many countries. There may be about 5 million regular cocaine users in the USA, and users who have significant difficulties, including serious as well as fatal medical complications, continue to be reported frequently [ , ]. Deaths occur not only from overdosage but also from drug-induced mental states, which can lead to serious injuries [ ].
Cocaine was also the first aminoester local anesthetic, and its adverse effects differ from those of other local anesthetics. Owing to its rapid absorption by mucous membranes, cocaine applied topically can cause systemic toxic effects. There is a wide variation in the rate and amount of cocaine that is systemically absorbed. This variability can be affected by the type and concentration of vasoconstrictor used with cocaine and also accounts for the differences in cocaine pharmacokinetics in cocaine abusers [ ].
As a recreational drug cocaine can be snorted (sniffed), swallowed, injected, or smoked. The street drug comes in the form of a white powder, cocaine hydrochloride. The hydrochloride salt and the cutting agents are removed to create the free base, which is smoked. The inexpensive widely available crack formulation is prepared by alkalinizing cocaine hydrochloride and precipitating the resultant alkaloidal free-base cocaine, which, unlike the hydrochloride, is not destroyed by heat when smoked. Smoking crack provides a rapid effect, comparable to that of intravenous injection. Intense euphoria, followed within minutes by dysphoria, leads to frequent dosing and a greater potential for rapid addiction [ ]. As with amphetamines, the euphoric effect can enhance craving, and repeated reinforcement can lead to conditioned drug responses, which facilitate dependence. Facilitated conditioned effects with cocaine may be due to its rapid elimination and the development of acute tolerance. Frequent repeated dosing becomes necessary to sustain euphoria, thereby promoting a tight temporal juxtaposition of euphoria with recent drug-taking [ ].
Rapid intravenous or inhalational administration of cocaine can cause very high concentrations in areas of high vascular perfusion, for example the heart and brain, before eventual distribution to other tissues. Under these conditions there is a catecholaminergic storm in the heart and a local anesthetic effect, with prolongation of conduction. Once beyond the immediate period of vulnerability, accumulation (for example through frequent overdosing or accidents from body packing of condom-filled stimulants to avoid detection) leads to a different cascade of events over a period of hours, leading to death. This cascade includes a catecholaminergic hypermetabolic state, with hyperpyrexia and acidosis, anorexia, and repeated seizures, usually ending in cardiac collapse [ , ]. On the other hand, chronic dosing can cause catecholaminergic cardiomyopathy, for example contraction bands, cardiomegaly [ , ], and repeated vasospastic insults to cerebral and coronary arteries [ ]. Whether these chronic effects predispose to increased sensitivity to acute toxicity has not been systematically explored, but autopsy studies suggest that they do.
In addition to other chronic changes in abusers, personality deterioration carries a significant association with high-risk behaviors, which are a source of physical and psychiatric morbidity and mortality. These include suicide, violent trauma and aggressive behavior, high-risk methods of drug use (for example needle sharing), and high-risk sexual behavior, with increased risks of HIV, hepatitis B, and other infections.
The stimulant properties of cocaine are similar to those of amphetamines, although the differences are notable, in part because of the very short half-life of cocaine. However, cocaine has the same problem of abuse potential as other stimulants, and at high doses causes stimulant psychosis [ ]. In addition, even when it is used as a local nasopharyngeal anesthetic, it has toxic, even fatal, effects in high doses.
Death from cocaine often occurs within 2–3 minutes, suggesting direct cardiac toxicity, fatal dysrhythmias, and depression of medullary respiratory centers as common causes of death [ , ]. Thus, cocaine’s local anesthetic properties can contribute additional hazards when high doses are used, reminiscent of deaths reported in the era when it was used as a mucous membrane paste for nasopharyngeal surgery [ ].
Periods of increased cocaine use, especially intravenous administration, inhalation of the free base, and high-dose use, are associated with cocaine-related deaths. For example, according to the Drug Abuse Warning Network, there was a three-fold increase in such deaths from 195 to 580 per year in the USA between 1981 and 1985. Despite the importance of these mortality data, relatively little is known of the types of pathophysiological sequences involved in the cascade of events leading to death. More important, there is a paucity of guidelines to appropriate diagnostic and treatment strategies for the various prefatal conditions.
Patients with brain death due to acute intoxication of drugs, including cocaine, can be organ donors. Satisfactory outcomes of graft functions were achieved in orthotopic liver transplants [ , ], kidney transplants [ ], and lung transplants [ ]. The use of grafts from donors with brain death due to cocaine overdosage may be a valid option to expand the donor pool and help maximize the availability of scarce donor organs.
Cocaine initially causes excitement and euphoria. Later, with higher doses, lower centers become involved, producing reduced coordination, tremors, hyper-reflexia, increased respiratory rate, and at times nausea, vomiting, and convulsions. These symptoms are eventually followed by CNS depression.
Cardiovascular effects include tachycardia, hypertension, and increased cardiac irritability; large intravenous doses can cause cardiac failure. Cardiac dysrhythmias have been ascribed to a direct toxic effect of cocaine and a secondary sensitization of ventricular tissue to catecholamines [ ], along with slowed cardiac conduction secondary to local anesthetic effects. Myocardial infarction has increased as a complication of cocaine abuse [ , ]. Dilated cardiomyopathies, with subsequent recurrent myocardial infarction, have been associated with long-term use of cocaine, raising the possibility of chronic effects on the heart [ ]. Many victims have evidence of pre-existing fixed coronary artery disease precipitated by cocaine [ ]. However, myocardial infarction has been noted even in young intranasal users with no evidence of coronary disease [ ], defined by autopsy or angiography [ , ]. If applied to mucous membranes, cocaine causes local vasoconstriction and, with chronic use, necrosis.
As a general rule, mortality is higher when cocaine is used intravenously or as smoked free base than if taken nasally or orally [ ]. The symptoms of acute cocaine poisoning include agitation, sweating, tachycardia, tonic-clonic seizures, severe respiratory and metabolic acidosis, apnea, and ventricular dysrhythmias. Seizures occur at high doses, and may be a major determinant of fatal outcomes; their control with sedatives is important to reduce lethality [ ]. Associated hyperthermia can contribute as a primary cause in cases of fatal hyperpyrexia, and can potentiate the hypoxic cardiovascular events in cardiac deaths in those who survive the initial acute dose [ , ]. A study of a very large number of cocaine deaths showed that the morbidity rate increased by four times on days on which the ambient temperature rose above 31.1 °C [ ]. The final agonal events in cocaine deaths involve the combination of sympathomimetic myocardial responses and/or cardiac conduction slowing, secondary to cocaine’s local anesthetic effect, leading to dysrhythmias [ ]. In reported fatal overdoses, convulsions and death have usually occurred within minutes. Most patients who have survived for the first 3 hours after an initial acute overdose have been likely to recover. Treatment includes respiratory and cardiovascular resuscitative measures. Short-acting barbiturates, benzodiazepines, beta-blockers, and phentolamine have all been used with some success [ , ]. Because of a possible risk of coronary vasodilatation with the use of propranolol to manage dysrhythmias in cocaine overdose, the use of labetalol for this indication is recommended, if a beta-blocker is required [ , ]. In one study of 60 cocaine-related deaths, autopsy findings were non-specific but typical of those found in respiratory depression of central origin [ ].
Cocaine abuse is a risk factor for myocardial ischemia, infarction, and dysrhythmias, as well as pulmonary edema, ruptured aortic aneurysm, infectious endocarditis, vascular thrombosis, myocarditis, and dilated cardiomyopathy [ ].
The cardiovascular toxicity of cocaine has been reviewed, providing an explanation of its ischemic and prothrombotic effects, accelerated coronary atherosclerosis, coronary vasoconstriction, and dysrhythmias, including sinus tachycardia or bradycardia, ventricular fibrillation, ventricular tachycardia, prolongation of the QTc interval, and torsade de pointes [ ].
Cocaine and its metabolite block sodium and potassium channels and its use is also associated with QT interval prolongation.
A 37-year-old man developed severe chest pain and shortness of breath after smoking 200 + rocks of crack cocaine in 72 hours while attempting to walk several miles in order to get more drug money [ ]. His symptoms resolved before the ambulance arrived. His medical history included a prior episode of chest pain after a 3-day crack cocaine binge. He also occasionally used alcohol and marijuana. He was anxious but had a normal heart rate and blood pressure. His drug screen showed cocaine. Cardiac enzymes were within the reference ranges. An initial electrocardiogram showed a QTc interval of 621 ms which shortened to 605 ms after 2 hours, 530 ms after 7 hours, and 543 ms after 15 hours. One month later the QTc interval was 453 ms.
Prolongation of the QT interval after cocaine use has previously been reported to resolve within 72 hours, but a case in which it persisted for 5 days has been reported [ ].
A 59-year-old African–American woman developed syncope after using cocaine for 4 days. She had bradycardia, a left-sided carotid bruit, and a QTc interval of 600 msec, which was attributed to cocaine. She was taking no medications known to prolong the QT interval and she had no family history of similar events. Serial cardiac enzymes were within the reference ranges. Two-dimensional echocardiography showed left ventricular hypertrophy and normal left ventricular systolic function. Carotid angiography showed 90% stenosis in the left internal carotid artery.
The authors attributed the syncopal episode to cocaine-associated dysrhythmias. They speculated that cocaine affects cardiovascular physiology by preventing synaptic reuptake of noradrenaline; it also acts like a type 1 antidysrhythmic agent, inhibiting membrane repolarization and preventing cardiac repolarization by blocking potassium channels.
The effect of cocaine on QT variability, a measure of cardiac repolarization has been assessed in 29 healthy volunteers with past cocaine exposure, who received randomized sequential intravenous infusions of cocaine 20 mg and 40 mg or placebo; 17 repeated the course 1 week later [ ]. Cocaine significantly and dose-dependently increased QT variability; the results were reproducible 1 week later.
In 14 habitual cocaine users, who smoked cocaine 25 mg during closely monitored 12-minute sessions with electrocardiographic recording, the heart rate increased by a mean of 22/minute; there was significant QTc prolongation, reduced T wave amplitude, and increased U wave amplitude [ ]. One patient developed an accelerated junctional rhythm and five had non-specific ST-T wave abnormalities.
The authors of these papers discussed how cocaine has a destabilizing effect on cardiac repolarization, which contributes to cardiac dysrhythmias. Like other sympathomimetic agents, cocaine is an α1, β1, and β2 adrenoceptor agonist with sodium channel blocking properties.
A Brugada-like syndrome has been reported in a patient with cocaine toxicity and was initially interpreted as acute ST-segment elevation myocardial infarction [ ]. The authors postulated that the electrocardiographic changes may have been due to uncovering by cocaine of an underlying genetic predisposition or because of a direct effect on cardiac sodium channels.
A 36-year-old man became comatose 14 hours after inhaling an unspecified amount of heroin for an unspecified duration [ ]. He had been taking lithium, chlorpromazine, and diazepam for a chronic psychosis. His family reported recreational use of drugs of abuse. His Glasgow coma score was 4 without focal deficits. A toxicology screen was positive for cocaine and remained positive for 4 days after admission. His electrocardiogram showed prominent coved ST elevation and J wave amplitude of at least 2 mm in leads V1–V 3, followed by negative T waves with no isoelectric separation, associated with right incomplete bundle branch block indicative of type 1 Brugada syndrome. His serum potassium concentration was 5.9 mmol/l. He was immediately treated with 42% sodium bicarbonate intravenously. The Brugada pattern completely resolved in 24 hours and his creatinine improved significantly in 48 hours. Transthoracic echocardiography was normal.
The authors of this report thought that the Brugada syndrome was probably not due to chlorpromazine or lithium in this patient, and it had not been previously described with heroin. It may have been due to hyperkalemia (as the Brugada pattern normalized when the serum potassium concentration normalized), perhaps facilitated by cocaine. Another case of Brugada syndrome is described under “Drug overdose”.
Dysrhythmias seem to be the most likely cause of sudden death from cocaine, but cardiac conduction disorders are more common in patients with acute cocaine toxicity. Severe cocaine toxicity also causes acidemia and cardiac dysfunction [ ]. Four patients developed seizures, psychomotor agitation, and cardiopulmonary arrest; two of these are briefly summarized here.
A 43-year-old man injected a large dose of cocaine in a suicide attempt and had a seizure and cardiopulmonary arrest, from which he was resuscitated. His arterial blood pH was 6.72 and his electrocardiogram showed a wide complex tachycardia. An infusion of sodium bicarbonate maintained the blood pH at 7.50 and the electrocardiogram became normal. The bicarbonate infusion was discontinued after 12 hours.
A 25-year-old man had a cardiac arrest after taking one “knot” or sealed bag of crack cocaine (2.5 g) and was resuscitated. His arterial blood pH was 6.92 and an electrocardiogram showed sinus rhythm, QRS axis 300°, and terminal 40 msec of the QRS axis 285°. After an infusion of sodium bicarbonate, his blood pH was 7.30, his QRS axis 15°, and the terminal 40 msec QRS axis 30°. He passed the bag of cocaine rectally within 12 hours of admission.
These patients’ initial laboratory values showed acidosis, prolongation of the QRS complex and QTc interval, and right axis deviation. Appropriate treatment included hyperventilation, sedation, active cooling, and sodium bicarbonate, which led to correction of the blood pH and of the cardiac conduction disorders. The authors suggested that when intracellular pH is lowered, myocardial contractility is depressed as a result of reduced calcium availability. During acidosis, there are abnormalities of repolarization and depolarization, which potentiate dysrhythmias.
The electrocardiographic PR interval increased with increasing abstinence from crack cocaine in a study of 441 chronic cocaine users who had smoked at least 10 g of cocaine in the 3 months before enrollment [ ]. The authors suggested that this may have reflected the normalization of a depolarization defect. Chronic cocaine users have shortened PR intervals, indicative of rapid cardiac depolarization.
Pregnancy increases the incidence of dysrhythmias in patients with Wolff–Parkinson–White syndrome. This association may relate to an effect of estrogen, increased plasma volume, or increased maternal stress or anxiety.
Neonatal heart rate and heart rate variability are used to detect a variety of pathophysiological alterations in the autonomic nervous system regulation of cardiac function. The effects of prenatal cocaine exposure on heart rate and heart rate variability have been studied in the presence of orthostatic stress in near-term and full-term neonates [ ]. Infants with prenatal cocaine exposure (n = 21) and controls (n = 23) were enrolled within 120 hours of birth. An electrocardiogram was recorded for 1 hour during quiet sleep, for 30 minutes supine, and then for 30 minutes in an inclined position. Compared with controls, the infants with prenatal cocaine exposure had a delayed and prolonged reaction to orthostatic stress. The results suggested that the effects of prenatal cocaine exposure on the development of the sympathetic and parasympathetic nervous systems could alter cardiovascular function.
Therapeutic hypothermia has been successfully used in cocaine-induced cardiac arrest [ ].
A 28-year-old woman had a cardiac arrest with pulseless electrical activity after consuming cocaine. Despite treatment with chest compression, adrenaline, and atropine or vasopressin, she had two further episodes of pulseless electrical activity and was given an infusion of noradrenaline. When her vital signs returned, she was comatose with ventricular fibrillation. Hypothermia was initiated, lowering her body temperature from 34.7oC (94.4o F) to 32–33o C (89.6–91.4o F) for 24 hours. She was then gradually re-warmed over 6 hours and was extubated. She had near-complete neurological recovery with mild deficits in short-term memory and orientation.
Although hypothermia has been shown to be a positive intervention in comatose survivors of cardiac arrest, drug overdose was an exclusion criterion in previous studies.
By one estimate, since the first report in 1982, over 250 cases of myocardial infarction due to cocaine have been reported, mostly in the USA. The first report from the UK was published in 1999 [ ]. Acutely, cocaine suppresses myocardial contractility, reduces coronary caliber and coronary blood flow, induces electrical abnormalities in the heart, and increases heart rate and blood pressure. These effects can lead to myocardial ischemia [ , ]. However, intranasal cocaine in doses used medicinally or recreationally does not have a deleterious effect on intracardiac pressures or left ventricular performance [ ]. The EIDOS and DoTS descriptions of cocaine-induced myocardial ischemia are shown in Figure 1 .
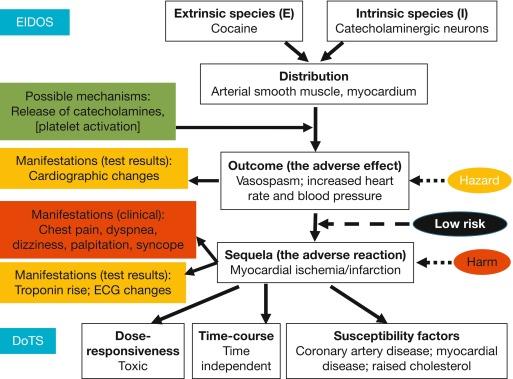
As cocaine use has become more widespread, the number of cocaine-related cardiovascular events has increased [ ]. Myocardial ischemia and infarction associated with cocaine are unrelated to the route of administration, the amount taken, and the frequency of use. The risk of acute myocardial infarction is increased after acute use of cocaine and it can occur in individuals with normal coronary arteries at angiography. The patients are typically young men and smokers and do not have other risk factors for atherosclerosis.
Possible predictors of cardiovascular responses to smoked cocaine have been studied in 62 crack cocaine users (24 women and 38 men, aged 20–45 years) who used a single dose of smoked cocaine 0.4 mg/kg [ ]. Physiological responses to smoked cocaine, such as changes in heart rate and blood pressure, were monitored. The findings suggested that higher baseline blood pressure and heart rate, a greater amount and frequency of current cocaine use, and current cocaine snorting predicted a reduced cardiovascular response to cocaine. By contrast, factors such as male sex, African-American race, higher body weight, and current marijuana use were associated with a greater cardiovascular response.
A 26-year-old man reported smoking 10 cigarettes/day and using cocaine by inhalation at weekends [ ]. His electrocardiogram showed raised anterolateral ST segments. He had raised creatine kinase MB activity and troponin I concentration. He was normotensive with signs of pulmonary congestion. Ventriculography showed anterolateral and apical hypokinesia and an ejection fraction of 21%. Angiography showed massive thrombosis of the left anterior descending coronary artery. He was given recombinant tissue plasminogen activator by intravenous infusion. Angiography 4 days later showed thrombus resolution, and ventriculography showed an improved ejection fraction. He was symptom-free 6 months later.
A 50-year-old man had 12 hours of chest pain and shortness of breath after a cocaine binge. His history included hyperlipidemia, cigarette smoking, and cocaine use [ ]. The troponin concentration was increased, at 25 ng/ml. An electrocardiogram showed anteroseptal ST segment elevation and inferolateral depression with T wave inversion. An angiogram showed multi-vessel occlusion of the left anterior descending artery, right coronary artery, and left circumflex artery. Ventriculography showed severe anteroapical and inferior wall hypokinesis with an ejection fraction of 25%. Angioplasty was performed urgently and final angiography showed no residual stenosis in the treated vessels.
In a cocaine abuser, resolution of intracoronary thrombosis with direct thrombin has been successfully attempted [ ]. Medical treatment was started with tirofiban and low molecular weight heparin, and 48 hours later they were replaced with bivalirudin, a direct thrombin inhibitor, in an initial bolus dose of 0.1 mg/kg followed by an infusion of 0.25 mg/kg/hour. Repeat angiography 48 hours after bivalirudin showed near total thrombus dissolution with resolution of the electrocardiographic abnormalities.
Fiberoptic bronchoscopy is often done after intratracheal injection of 2.5% cocaine solution and lidocaine spray. Acute myocardial infarction after fiberoptic bronchoscopy with intratracheal cocaine has been reported [ ].
A 73-year-old man with a history of breathlessness, cough, and weight loss had some ill-defined peripheral shadow in the upper zones of a chest X-ray. He had fiberoptic bronchoscopy with cocaine and lidocaine and 5 minutes later became distressed, with dyspnea, chest pain, and tachycardia. Electrocardiography showed an evolving anterior myocardial infarction. Coronary angiography showed a stenosis of less than 25% in the proximal left anterior descending artery with coronary artery spasm. He made an uneventful recovery.
The authors suggested that the principal cardiac effects of cocaine can be attributed to or are mediated by the following mechanisms: increased myocardial oxygen demand due to an acute rise in systemic blood pressure and heart rate; coronary vasoconstriction caused by alpha-adrenergic effects and calcium-dependent direct vasoconstriction; and promotion of arteriosclerosis and endothelial dysfunction, which predisposes to vasoconstriction and thrombosis.
Cocaine-induced myocardial ischemia in pregnancy mistaken for Wolff–Parkinson–White syndrome has been reported [ ].
A 22-year-old pregnant woman at 38 weeks’ gestation developed chest pain, palpitation, and shortness of breath. Her blood pressure was 148/97 mmHg, heart rate 105/minute, and respiratory rate 22/minute. An electrocardiogram showed a short PR interval and a broad QRS complex with slurred upstrokes of the R waves in leads V2 and V3. Other laboratory studies were normal. The fetal heart rate was 160/minute. The differential diagnosis included a new onset dysrhythmia, Wolff–Parkinson–White syndrome, and myocardial ischemia/infarction. With supportive treatment and monitoring the dysrhythmia resolved. Serial creatine kinase measurements were normal. However, there were cocaine metabolites in the urine. The patient admitted to having used cocaine 3–4 hours before the episode.
The use of cocaine among women of reproductive age is increasing and pregnancy also enhances the potential cardiovascular toxicity of cocaine.
Fatal pulmonary edema developed in a 36-year-old man shortly after injecting free-base cocaine intravenously [ ].
Cocaine users often present with complaints suggestive of acute cardiac ischemia (chest pain, dyspnea, syncope, dizziness, and palpitation). The risk of actual acute cardiac ischemia among cocaine users with such symptoms is low. In a review of the clinical database from the Acute Cardiac Ischemia–Time Insensitive Predictive Instrument Clinical Trial, a multicenter prospective clinical trial conducted in the USA, 293 (2.7%) of 10 689 enrolled patients, had cocaine-associated complaints, but the rate varied from 0.3% to 8.4% in the 10 participating hospitals [ ]. Only six of these patients had a diagnosis of acute cardiac ischemia (2.0%), four with unstable angina and two with acute myocardial infarction. The cocaine users were admitted to the coronary care unit as often as other study participants (14 versus 18%), but were much less likely to have confirmed unstable angina (1.4 versus 9.3%). A second study also suggested that cocaine users who present with chest pain have a very low risk of adverse cardiac events [ ]. Emergency departments have instituted centers for the evaluation and treatment of patients with chest pain who are at low to moderate risk of acute coronary syndromes. In this particular study, patients with a history of coronary artery disease or presentations that included hemodynamic instability, electrocardiographic changes consistent with ischemia, or clinically unstable angina were directly admitted to hospital. In a retrospective study of 179 patients with reliable 30-day follow-up in chest pain centers, there was one cardiac complication due to cocaine use.
Frequency : During the hour after cocaine is used, the risk of myocardial infarction is 24 times the baseline risk [ ]. Cocaine users have a lifetime risk of non-fatal myocardial infarction that is seven times the risk in non-users, and cocaine use accounts for up to 25% of cases of acute myocardial infarction in patients aged 18–45 years [ ]. In 2000, there were 175 000 cocaine-related visits to emergency departments in the USA [ ], with chest discomfort in 40% of the patients [ ], 57% of whom were admitted to the hospital and had an admission lasting an average of 3 days [ ], involving huge costs [ ].
Based on a retrospective study of 344 patients with cocaine-associated chest pain, it has been suggested that patients who do not have evidence of ischemia or cardiovascular complications over 9–12 hours in a chest-pain observation unit have a very low risk of death or myocardial infarction during the 30 days after discharge [ ]. Nevertheless, patients with cocaine-associated chest pain should be evaluated for potential acute coronary syndromes; those who do not have recurrent symptoms, increased concentrations of markers of myocardial necrosis, or dysrhythmias can be safely discharged after 9–12 hours of observation. A protocol of this sort should incorporate strategies for treating substance abuse, since there is an increased likelihood of non-fatal myocardial infarction in patients who continue to use cocaine.
The incidence of acute myocardial infarction in cocaine-associated chest pain is small but significant [ ]. The electrocardiogram has a higher false-positive rate in these patients. A normal electrocardiogram reduces the likelihood of myocardial injury but does not exclude it.
Cocaine use may account for up to 25% of acute myocardial infarctions among patients aged 18–45 years. The safety of a 12-hour observation period in a chest pain unit followed by discharge in individuals with cocaine-associated chest discomfort who are at low risk of cardiovascular events has been evaluated in 302 consecutive patients aged 18 years or older (66% men, 70% black, 84% tobacco users) who developed chest pain within 1 week of cocaine use or who tested positive for cocaine [ ]. Cocaine use was self-reported by 247 of the 302 subjects and rest had urine positive for cocaine; 203 had used crack cocaine, 51 reported snorting, and 10 had used it intravenously. Of the 247 who reported cocaine use, 237 (96%) said they had used it in the week before presentation and 169 (68%) within 24 hours before presentation. Follow-up information was obtained for 300 subjects. There were no deaths from cardiovascular causes. Four patients had a non-fatal myocardial infarction during the 30-day period; all four had continued to use cocaine. Of the 42 who were directly admitted to hospital, 20 had acute coronary syndrome. The authors suggested that in this group of subjects, observation for 9–12 hours with follow-up is appropriate.
Mechanisms and pathophysiology : Cocaine can cause myocardial infarction by multiple mechanisms, including coronary vasoconstriction, increasing heart rate and blood pressure, platelet activation, and possibly accelerated atherosclerosis [ , ]. The incidence of myocardial infarction after cocaine ingestion is estimated to be 0.7–6%. Evaluation of cocaine-associated chest pain is similar to evaluation in those who do not use cocaine; however, because cocaine can cause rhabdomyolysis, creatine kinase activity is not as reliable as other tests [ ]. TIMI risk scores, which are used to predict 14-day mortality in unstable angina and non-ST-elevation myocardial infarction, are not helpful in predicting outcomes in those with cocaine-associated chest pain. In a prospective cohort study of 261 patients with cocaine-associated chest pain, TIMI scores did not correlate with acute myocardial infarction, revascularization, or death in the 30 days from the index visit [ ].
Tachycardia and vasoconstriction from cocaine can exacerbate coronary insufficiency, complicated by dysrhythmias and hypertensive and vascular hemorrhage [ ]. Sudden deaths have been reported in patients with angina [ ]. Chronic dosing includes cardiomyopathy and cardiomegaly; other chronic conditions include endocarditis and thrombophlebitis. Crack smoking has led to pneumopericardium [ ].
The cardiac effects of intracoronary infusion of cocaine have been studied in dogs and humans [ ]. The procedure can be performed safely and does not alter coronary arterial blood flow. The effects of direct intracoronary infusion of cocaine on left ventricle systolic and diastolic performance have been studied in 20 patients referred for cardiac catheterization for evaluation of chest pain. They were given saline or cocaine hydrochloride (1 mg/minute) in 15-minute intracoronary infusions, and cardiac measurements were made during the final 2–3 minutes of each infusion. The blood cocaine concentration obtained from the coronary sinus was 3.0 μg/ml, which is similar in magnitude to the blood–cocaine concentration reported in abusers who die of cocaine intoxication. Minimal systemic effects were produced. The overall results were that cocaine caused measurable deterioration of left ventricular systolic and diastolic performance.
Accelerated extensive atherosclerosis secondary to chronic cocaine abuse has also been reported [ ].
A 32-year-old man, who was a cigarette smoker (10 cigarettes/day) and had been a frequent cocaine user for 16 years, developed acute chest pain 2 hours after heavy cocaine use. He had electrocardiographic abnormalities consistent with an acute myocardial infarction, confirmed by serial enzyme measurements. There was no family history of atherosclerosis. Echocardiography showed a large akinetic anteroapical segment. Serum lipoprotein concentrations were normal. Despite management with aspirin, glyceryl trinitrate, heparin, and morphine, he required emergency cardiac catheterization because of prolonged chest pain. Coronary angiography showed severe atherosclerosis of the middle and distal segments of the left anterior descending coronary artery and a large diagonal branch. Intracoronary glyceryl trinitrate and nitroprusside ruled out coronary spasm. Intravascular ultrasound localized the lesion to the middle of the left anterior descending artery and showed diffuse plaques along the entire artery, with variable composition, including fibrocalcific changes consistent with a chronic process. Two overlapping stents were inserted.
The authors acknowledged that the mechanisms for accelerated atherosclerotic process are not known. However, they cited research that suggests that cocaine has pro-atherogenic effects in blood vessels.
The prevalence of coronary atherosclerosis has been described in 51 patients (49 men) aged under 50 years with an acute persistent ST-segment elevation myocardial infarction with and without cocaine exposure who were admitted to a US hospital [ ]; 17 patients (16 men) self-reported cocaine use more than 10 times in a lifetime and 35 were non-cocaine controls. Coronary angiography showed that cocaine users had a higher prevalence of atherosclerosis (65%) and a higher number of coronary artery lesions (2.3 per patient) compared with controls (32% and 1.6 per patient). Cocaine users had a lower mean age and a lower mean number of cardiac risk factors. The authors challenged a popular explanation that spasm in otherwise normal coronary arteries is the main cause of myocardial infarction in young cocaine users. Their findings strongly support a proatherosclerotic effect of cocaine.
The hypothesis that cocaine users have increased coronary microvascular resistance, even in the absence of recent myocardial infarction, coronary artery disease, or spasm, has been assessed in 59 consecutive cocaine users without acute or recent myocardial infarction or angiographically significant epicardial stenosis or spasm [ ]. Microvascular resistance was significantly increased by 26–54% in cocaine users. There was an abnormally high resistance in the left anterior descending artery in 61% of the patients, in the left circumflex artery in 69%, and in the right coronary artery in47%. Increased microvascular resistance may explain many important cardiovascular effects of cocaine and has therapeutic implications. For example, slow coronary filling in diagnostic tests may suggest the possibility of cocaine use in patients in whom it was not otherwise suspected. There was increased microvascular resistance in the coronary bed even after the acute effects of a dose of cocaine would have worn off, suggesting that cocaine may have long-lasting effects on coronary microvasculature. This implies that medical therapy of vasoconstriction should be continued for extended periods. Moreover, heightened microvascular resistance in cocaine users may explain the development of chest pain and myocardial ischemia in patients who do not have epicardial stenosis due to coronary artery disease or spasm. The authors suggested that in the absence of coronary artery disease, the small vessel effects of cocaine may be more important. As the process is diffuse rather than confined to one vascular territory, electrocardiographic findings may not be localizing.
In a report of cocaine-associated chest pain, the authors studied the incidence and predictors of underlying significant coronary disease in 90 patients with and without myocardial infarction [ ]. Patients with 50% or more stenosis of coronary arteries or major branches or bypass graft were included and 50% of them had significant disease: one-vessel disease in 32%, two-vessel disease in 10%, three-vessel disease in 6%, and significant graft stenosis in 3%. There was significant disease in 77% of patients with myocardial infarction or a raised troponin I concentration, compared with 35% of patients without myonecrosis. Predictors of significant coronary disease included myocardial damage, a prior history of coronary disease, and a raised cholesterol. Only seven of the 39 patients without myonecrosis or a history of coronary disease had significant angiographic disease. The authors concluded that significant disease is found in most patients with cocaine-associated myocardial damage. In contrast, only a minority of those without myonecrosis have significant coronary disease.
In a review of 114 cases, coronary anatomy, defined either by angiography or autopsy, was normal in 38% of chronic cocaine users who had had a myocardial infarction [ ]. The authors of another review concluded that “the vast majority of patients dying with cocaine toxicity, either have no pathological changes in the heart, or only minimal changes” [ ]. There can be a delay between the use of cocaine and the development of chest pain [ ]. The results of a study of 101 consecutive patients admitted with acute chest pain related to cocaine suggested that it commonly causes chest pain that may not be secondary to myocardial ischemia [ ]. The use of intranasal cocaine for therapeutic purposes (to treat epistaxis) was associated with myocardial infarction in a 57-year-old man with hypertension and stable angina [ ].
Of 91 patients with cocaine-induced myocardial infarction identified from a literature review, 44 had used cocaine intranasally, 27 had smoked it, and 19 had used it intravenously; two-thirds had their myocardial infarction within 3 hours of use [ ]. Almost half had a prior episode of chest pain. There were acute complications related to the myocardial infarction in 18. Of 24 patients followed up, 58% had subsequent cardiac complications.
Two-phase myocardial imaging with 99mTc-sestamibi can be helpful in the definitive diagnosis of cocaine-induced myocardial infarction in patients with a history of cocaine use, chest pain, and a non-diagnostic electrocardiogram [ ]. Damage to the myocardium associated with cocaine can be unrecognized by the abuser [ ]. Major electrocardiographic findings (including myocardial infarction, myocardial ischemia, and bundle branch block) were recorded during a review of 99 electrocardiograms of known cocaine abusers. None of the 11 patients with major electrocardiographic changes had a past history of cardiac disease or had complained of chest pain. The mechanism by which cocaine causes acute myocardial damage is unclear. Of 20 healthy cocaine abusers given intravenous cocaine, in doses commonly self-administered, or placebo, none developed myocardial ischemia or ventricular dysfunction on two-dimensional echocardiography during the test [ ].
Myocardial infarction has been documented in 6% of patients who present to emergency departments with cocaine-associated chest pain [ , ]. Treatment of cocaine-associated myocardial infarction has previously generally been conservative, using benzodiazepines, aspirin, glyceryl trinitrate, calcium channel blockers, and thrombolytic drugs. In the context of 10 patients with cocaine-associated myocardial infarction, who were treated with percutaneous interventions, including angioplasty, stenting, and AngioJet mechanical extraction of thrombus, the authors suggested that percutaneous intervention can be performed in such patients safely and with a high degree of procedural success [ ]. Patients with cocaine-associated chest pain and electrocardiographic ST-segment elevation should first undergo coronary angiography, if available, followed by percutaneous intervention. Alternatively, thrombolytic drugs can be used. However, the relative safety and efficacy of thrombolytic drugs compared with percutaneous intervention is undefined in patients with cocaine-associated myocardial infarction.
Management : Although percutaneous revascularization for cocaine-associated myocardial infarction is the preferred method of treatment [ ], the feasibility and safety of multivessel primary angioplasty has been demonstrated. In patients with persistent myocardial ischemia despite medical therapy, or evidence of cardiogenic shock, aggressive early intervention is particularly beneficial. Beta-blockers should be avoided in patients who have recently used cocaine; they fail to control the heart rate, enhance cocaine-induced vasoconstriction, increase the likelihood of seizures, and reduce survival [ ].
There is a debate about the role of beta-blockers in treating cocaine-associated myocardial infarction. The American Heart Association's treatment guidelines [ ] and a review [ ] are consistent with the prior clinical convention that cautions against the use of beta-blockers, because of concern that the beta-blocking actions of cocaine on the coronary arteries will leave the alpha-adrenergic effects unopposed, leading to coronary vasospasm. This was based on an observation in 30 healthy adults that vasoconstriction after intranasal cocaine worsened after administration of a beta-blocker [ ]. A literature review of three case reports, two placebo-controlled trials, and three national guidelines did not find evidence or opinion favoring the use of beta-blockers after cocaine exposure [ ]. However, a retrospective cohort study has shown possible benefit of beta-blockers in reducing the risk of myocardial infarction after cocaine use, calling this into question [ ]. Records from 363 consecutive admissions (307 individual patients) to telemetry and intensive care units that had urine toxicology studies positive for cocaine were reviewed for the effects of beta-blockers on the development of myocardial infarction or death. Myocardial infarction was defined as a serum troponin concentration greater than 0.10 or ST elevation in two contiguous electrocardiogram leads. Patients who had received beta-blockers as out-patients and patients with missing troponin measurements were excluded, leaving 310 admissions and 296 patients. Beta-blockers were used in 33 cases. Two patients who received a beta-blocker developed a myocardial infarction and one died (incidence 1.7%), whereas 72 who did not receive a beta-blocker developed a myocardial infarction and 13 died (incidence 4.5%). Those who received a beta-blocker were more likely to have a history of heart failure and higher blood pressures and glucose concentrations, although these differences are more apt to increase the risk of myocardial infarction. This was a small study, and the results were somewhat limited by its retrospective design and because no data were available about the time of cocaine ingestion; however, it did provide preliminary information in favor of using beta-blockers. While some have called for a prospective randomized trial to evaluate beta-blockers in this setting, others have cautioned that this would be dangerous [ ].
In a randomized, controlled trial in 36 patients with cocaine-associated chest pain, the early use of lorazepam together with glyceryl trinitrate was more efficacious than glyceryl trinitrate alone in relieving the chest pain [ ]. These findings contrast with those of an earlier study in which there was no evidence of additional benefit from diazepam in managing cocaine-related chest pain [ ]. However, the Advanced Cardiovascular Life Support (ACLS) guidelines state that in cocaine-associated acute coronary syndromes a nitrate should be first-line therapy together with a benzodiazepine [ ].
Coronary artery dissection associated with cocaine is rare. The first case was reported in 1994 [ ] and a few other cases have been reported [ ].
A healthy 33-year-old man with prior cocaine use had a small myocardial infarction and, 36 hours later, having inhaled cocaine, developed a dissection of the left main coronary artery, extending distally to the left anterior descending and circumflex arteries. There was marked anterolateral and apical hypokinesis.
A 23-year-old man with a history of intravenous drug abuse and hepatitis C was found unconscious, hypoxic, and hypotensive. A urine drug screen was positive for cocaine metabolites, benzodiazepines, and opiates. An electrocardiogram suggested a myocardial infarction, verified by raised troponin I and the MB fraction of creatine kinase. He had severe hypokinesia with a left ventricular ejection fraction of 10%, falling to less than 5%. He became septic, developed multiorgan system failure, and died. The postmortem findings included dissection of the left anterior descending artery with complete occlusion of the true lumen and thrombosis of the false lumen. The left ventricle showed extensive transmural myocardial necrosis with adjacent contraction band necrosis. He also had deep vein thromboses in veins in the neck and abdomen and multiple pulmonary infarctions.
A 34-year-old woman developed chest pain suggestive of acute coronary syndrome, having inhaled cocaine 30 minutes before. Her blood pressure was 180/100 mmHg and an electrocardiogram showed sinus rhythm with anterolateral ischemia, ST segment depression, and T wave inversion. An echocardiogram showed a large hypokinetic area, including the middle and apical segments of the anterior septum and the anterior and lateral walls, and mild reduction in the left ventricular ejection fraction (45%). Troponin I and creatine kinase were slightly raised but the MB fraction was normal. Unstable angina was diagnosed but despite full medical therapy, the chest pain and ischemic changes did not resolve. Immediate catheterization showed a dissection flap within the left main trunk extending to the proximal portion of the descending anterior and circumflex arteries. There was no atherosclerosis in the coronary vessels. The flap resulted in a 90% stenosis of the proximal left anterior descending artery. Urgent coronary artery bypass surgery was successful.
Spontaneous coronary artery dissection is an unusual cause of acute coronary syndrome.
Aortic thrombus and renal infarction has been reported in a patient who used nasal cocaine [ ].
A 52-year-old woman with a history of hypertension for 15 years developed acute left flank pain, nausea, and vomiting. On a previous similar occasion 2 weeks before she had had a trace of proteinuria and microscopic hematuria. A contrast-enhanced CT scan of the abdomen had not shown stones, hydronephrosis, or morphological abnormalities. She had had no rash. Her urine contained cocaine. Creatine kinase and lactate dehydrogenase activities were raised and there was a leukocytosis. A second abdominal CT scan with contrast showed a segmental infarct of the left kidney. A transesophageal echocardiogram showed a 2 × 2 cm mobile mass, consistent with a thrombus, attached to the aortic arch, distal to the left subclavian artery. There was no evidence of atherosclerosis. She was given anticoagulants and aggressive fluid therapy for rhabdomyolysis.
The authors speculated that cocaine may have caused aortic inflammation by assuming that cocaine-related increased sympathetic tone along with possible cocaine-related aortic inflammation (similar to reported cases of cocaine-related vascular injury) may have led to enhance aggregation of platelets at the inflamed area, which would act as a nidus to form a thrombus. The thrombus resolved with anticoagulation within 13 days and similar results have been reported before [ ]. The patient had very high creatine kinase activity, suggesting rhabdomyolysis, which could have been caused by intense cocaine-related vasoconstriction.
Dissection of the aorta has been reported during cocaine use [ , ]. The authors of these two reports noted that all six cases of this rare complication reported in the past 5 years were in men with pre-existing essential hypertension. In a review of emergency visits to a hospital during a 20-year period, 14 of 38 cases of acute aortic dissection involved cocaine use; 6 were of type A and 8 of type B [ ]. Crack cocaine had been smoked in 13 cases and powder cocaine had been snorted in one case. The mean time of onset of chest pain was 12 hours after cocaine use. The chronicity of cocaine use was not known in most of the cases. The cocaine users were typically younger than the non-cocaine users. Chronic untreated hypertension and cigarette smoking were often present.
A 43-year-old man with untreated hypertension developed transient mild chest pressure followed by shortness of breath for 4 hours [ ]. He had long used tobacco, alcohol, and cocaine and admitted to having used cocaine within the last 12 hours. He had a tachycardia with a pansystolic murmur suggesting mitral regurgitation. Urine drug screen was positive for cocaine metabolites. A chest X-ray showed mild cardiomegaly and prominent upper lobe vasculature. An electrocardiogram showed atrial flutter at a rate of 130/minute and non-specific T wave changes. The diagnoses were myocardial infarction due to cocaine, with mild congestive heart failure, mitral regurgitation and atrial flutter. However, transesophageal echocardiography showed severe aortic insufficiency and a dissection flap in the ascending aorta. He underwent emergency repair of the aortic root and resuspension of the aortic valve.
Intramural hematoma of the ascending aorta has been reported in a cocaine user [ ].
A healthy 39-year-old man developed retrosternal chest pain radiating to the back with nausea and sweating. About 10–15 minutes before, he had inhaled cocaine for 2 hours and then smoked crack cocaine. He had an aortic dissection, which was repaired surgically.
The authors identified hypertension secondary to the use of cocaine as the risk factor for this complication.
Cocaine has been associated with aortic dissection, thought to be due to shearing forces from hypertension and tachycardia. In a retrospective chart review of 164 patients admitted with acute aortic dissection over 15 years, 16 patients (9.8%) had used powdered cocaine intranasally or had smoked crack within 24 hours before the onset of symptoms (by self-report or positive urine toxicology) [ ]. The mean time from drug use to symptoms was 13 hours. The cocaine users were significantly younger than those who had not used cocaine (mean age 47 versus 62 years). Outcomes, including length of hospital stay and mortality, were similar, with the exception of an increased rate of pulmonary complications in those who had used cocaine, postulated to be due to cocaine- or cigarette-induced pre-existing lung damage.
Fatal vasoconstriction of the aorta has been attributed to cocaine [ ].
After a 5-day crack cocaine binge, a 32-year-old woman developed severe bilateral leg pain and inability to walk. She drank several cans of beer to try to relieve the pain. She had a history of “speed balling”, or injecting a mix of heroin and cocaine. She was lethargic, with a systolic blood pressure of 70 mmHg, a heart rate of 172/minute, and a respiratory rate of 22/minute. Her truncal skin was mottled below the umbilicus. The abdomen was diffusely tender and no bowel sounds were detectable. Her limbs were mottled, pale, and cold to the touch. The femoral pulses were faintly audible with Doppler. There were no pulses distally. Sensation was markedly reduced in the legs. She was given oxygen, intravenous isotonic saline, and glyceryl trinitrate infusion. Her systolic blood pressure rose to 110 mmHg and her pulse rate to 110/minute. Abdominal ultrasound showed severe vasoconstriction of the abdominal aorta above the renal arteries. She had a raised creatinine kinase activity (56 000 mg/l), blood urea nitrogen of 156 mg/dl, creatinine of 6.2 mg/dl, and potassium of 4.0 mmol/l. On day 3, she had fever, acute septic shock, and disseminated intravascular coagulopathy and died.
Peripheral vascular disease in the fingers has been attributed to cocaine.
A 48-year-old man who smoked cigarettes and used cocaine developed ischemia of the right index finger due to occlusion of the distal ulnar artery [ ]. He had a history of recurrent deep vein thrombosis. A venous bypass graft was performed. Two years later he had non-healing gangrene of the left index finger. His blood pressure was normal in both arms. Urine toxicology was positive for cocaine. Angiography of the left arm showed small-vessel vasculitis.
A healthy 36-year-old man, who had used intranasal crack cocaine daily in increasing doses for 2 weeks, developed pain, numbness, swelling, and cyanosis of the fingers and toes aggravated by cold and an ulcer on one finger [ ]. Ultrasound Doppler of the hand confirmed ischemic finger necrosis. He was treated unsuccessfully with aspirin, diltiazem, and heparin, but responded to intravenous infusions of iloprost for 5 days.
Another less common complication of cocaine use is cerebral vasculitis [ ], and benign cocaine-induced cerebral angiopathy has been reported [ ]. (Stroke is discussed under the section Nervous system in this monograph).
The use of cocaine may be associated with higher risk of stent thrombosis after coronary stenting. Of 247 consecutive patients who received coronary stents, 12 were actively using cocaine (4.9%); four developed stent thrombosis; in three cases thrombosis occurred more than 30 days after stenting. Only two of the 235 patients without documented cocaine use (0.85%) had stent thrombosis during the same period [ ]. An accompanying editorial advised including cocaine among the risk factors for stent thrombosis and suggested that drug-eluting stents, which are associated with poorer endothelialization, should be avoided in cocaine users [ ].
Myocarditis has been attributed to cocaine [ ].
A 42-year-old healthy occasional user of cocaine developed fatigue, fever, cough, muscle aches, and abdominal pain after using cocaine 3 days before, and collapsed and died. Autopsy showed a mottled enlarged heart weighing 585 g and 50 ml of serous pericardial fluid. Histology of the myocardium showed a mononuclear inflammatory cell infiltrate with lymphocytes in and around the sinoatrial node and the bundle of His and the left bundle branch. Prominent contraction band necrosis, some coagulative necrosis, and myocyte loss were also present, and there was evidence of myocyte apoptosis. There were cocaine metabolites in the blood.
Before this report, cocaine-induced myocarditis has been associated solely with chronic use. The authors also pointed out that this is a first report of involvement of the bundle of His and left bundle branch. The mechanism of damage may include myocardial adrenergic stress and cardiac myocyte apoptosis.
Cardiovascular effects due to enhanced sympathetic activity include tachycardia, increased cardiac output, vasoconstriction, and increased arterial pressure. Myocardial infarction is the most common adverse cardiac effect [ ], and there is an increased risk of myocardial depression when amide-type local anesthetics, such as bupivacaine, levobupivacaine, lidocaine, or ropivacaine are administered with antidysrhythmic drugs.
A woman who inappropriately used cocaine on the nasal mucosa to treat epistaxis had a myocardial infarction [ ].
A patient who was treated with intranasal cocaine and phenylephrine during a general anesthetic had a myocardial infarction and a cardiac arrest due to ventricular fibrillation [ ].
Myocardial ischemia was reported in a fit 29-year-old patient after the nasal application of cocaine for surgery. No relief was gained from vasodilators or intracoronary verapamil, and there were no other signs of cocaine toxicity. Although coronary vasoconstriction and platelet activation are systemic effects of cocaine, pre-existing thrombus may also have played a part [ ].
Previous cocaine abuse has also been implicated in increasing the risk of myocardial ischemia when other local anesthetics are used.
Cardiac dysrhythmias have also been described in patients after the use of topical cocaine for nasal surgery [ ].
A patient who was treated with intranasal cocaine and submucosal lidocaine during general anesthesia developed ventricular fibrillation [ ].
These events do not appear to have been related to the concomitant use of a vasoconstrictor, but more to excessive doses of cocaine.
Substantial systemic absorption of cocaine can cause severe cardiovascular complications [ ].
An 18-year-old man had both nasal cavities prepared with a pack soaked in 3–5 ml of Brompton solution (3% cocaine, about 3 mg/kg, plus adrenaline 1:4000) 2 hours preoperatively. In the anesthetic room he was anxious and withdrawn, with a mild tachycardia. Ten minutes later the nasal pack was removed and polypectomy was begun, with immediate sinus tachycardia and marked ST depression on lead II of the electrocardiogram. Increasing the depth of anesthesia and giving fentanyl had little effect, and the procedure was terminated. After extubation a further electrocardiogram showed T wave flattening in leads II, III, aVF, and aVL. Further cardiac investigations ruled out a myocardial infarction, an anatomical defect, or other pathological or metabolic processes. On day 4 a stress electrocardiogram showed no ischemic changes.
Absorption of cocaine from the nasal mucosa in eight patients using cotton pledglets soaked in 4 ml of 4% cocaine and applied for 10 or 20 minutes resulted in an absorption rate four times higher than expected, but was not associated with any cardiovascular disturbance; however, one of four patients who received 4 ml of 10% cocaine for 20 minutes developed intraoperative hypertension and another transient ventricular tachycardia [ ]. The authors advised against topical use of 10% cocaine.
The respiratory effects of cocaine are well known [ ]. In healthy crack cocaine users, there is evidence of cocaine-related injury to the pulmonary microcirculation, from fiberoptic bronchoscopy and examination of the bronchoalveolar fluid in 10 cocaine-only smokers, six cocaine-plus-tobacco smokers, 10 tobacco smokers, and 10 non-smokers, all with normal respiratory function [ ]. The percentages of hemosiderin-positive alveolar macrophages (a marker of recent alveolar hemorrhage) were markedly increased in the cocaine smokers compared with the others. Furthermore, the concentrations of endothelin (ET-1), an indicator of cell damage, were significantly raised in the cocaine smokers and to a lesser extent in the cocaine-and-tobacco smokers. These findings suggest that many asymptomatic healthy crack users have chronic alveolar hemorrhage that is not clinically evident.
In 177 heavy cocaine users (compared with 75 non-cocaine users), some of whom were also tobacco or marijuana users, cocaine use was associated with a higher prevalence of acute respiratory symptoms, including black sputum and chest pain. However, chronic respiratory symptoms occurred at similar frequencies in both groups. In cocaine-only smokers, mild impairment in carbon monoxide diffusing capacity suggested pulmonary capillary membrane damage, and abnormal airway conductance suggested injury to the upper airway or the large intrathoracic bronchi. Reported pulmonary complications of crack cocaine range from acute symptoms (coughing, chest pain, and palpitation) to acute syndromes (end-stage lung disease, eosinophilic infiltrates of the lung, and pulmonary infarction). The single-breath carbon monoxide diffusing capacity after the use of crack cocaine was reduced in three of six reports [ ]. If confirmed, a reduced carbon monoxide diffusing capacity after crack may signify damage to the alveolar capillary membrane or the pulmonary vasculature. It has been suggested that a well-designed controlled study to investigate the true impact of crack on the lung is necessary, since several confounding factors may account for the discrepancy in results [ ].
Reports of acute pulmonary syndromes after the inhalation of cocaine have long been familiar.
A 32-year-old woman rapidly developed progressive deterioration of respiratory function leading to end-stage lung disease [ ]. An open lung biopsy showed an inflammatory process with extensive accumulation of free silica.
The authors cautioned that some cocaine may contain silica, which could lead to severe pulmonary complications after smoking.
A 27-year-old man twice developed inflammatory lung disease (with a predominance of eosinophils) after inhaling crack cocaine [ ]. Glucocorticoid treatment led to prompt resolution on both occasions.
A 23-year-old woman developed pulmonary infarction associated with the use of crack [ ].
Passive inhalation of free-base cocaine in small children can lead to serious consequences.
A previously healthy 3-week-old boy developed pulmonary edema and autonomic manifestations of cocaine exposure from passive use [ ]. The urinary drug screen was positive for benzoylecgonine, a cocaine metabolite.
Fatal pulmonary edema developed in a 36-year-old man shortly after he injected free-base cocaine intravenously [ ].
Severe bullous emphysema in a cocaine smoker has been described [ ].
A 40-year-old man with cough, shortness of breath, and fever progressed to respiratory failure. He had smoked cocaine for the previous 17 years. His tobacco history was not known. His medical history included recurrent respiratory tract infections. A chest X-ray and CT scan showed findings consistent with bilateral bullous emphysema with a right lung abscess. He was ventilated and given antibiotics but died from respiratory failure secondary to pneumonia. Sputum cultures were positive for Enterobacter cloacae and Streptococcus species. Alpha-1 antitrypsin deficiency was ruled out.
Spontaneous pneumomediastinum and pneumothorax have been reported [ ].
A 20-year-old obese Hispanic man awoke with severe, continuous retrosternal chest pain radiating to the neck and back [ ]. The pain was aggravated by deep breathing and local chest pressure. He denied substance abuse and gave a history of a flu-like illness 2 months before. His respiratory rate was 19/minute. He had a two-component pericardial rub. Laboratory blood testing ruled out myocardial infarction. His arterial blood gases and pH, electrocardiogram, chest X-ray, and echocardiogram were unremarkable. A later chest X-ray showed air in the mediastinum and chest CT confirmed the diagnosis of pneumomediastinum. Urine toxicology was positive for cocaine and cannabinoids. On further questioning, he admitted to substance use and performing a Valsalva maneuver during inhalation.
A 22-year-old previously healthy man presented with acute sore throat awakening him from sleep [ ]. He had palpable crepitation due to extensive cervical subcutaneous emphysema. A chest X-ray showed a pneumomediastinum and bilateral apical pneumothoraces. CT scan did not show any underlying lung disease. Bronchoscopy was unremarkable except for a swollen nasal mucosa and acute bronchitis. Bronchoalveolar lavage was normal without evidence of infection. The patient reported repeated cocaine consumption. No tube drainage was necessary and the air collections resolved spontaneously within days.
Crack, the heat stable form of cocaine, when smoked and followed by deep inhalation plus a Valsalva maneuver to increase uptake, and cough triggered by the sniffed substance can cause pulmonary barotrauma. The increased intra-alveolar pressure can cause alveolar rupture, with consequent air dissection through the peribronchial connective tissue in the mediastinum, pleural space, pericardium, peritoneum, or subcutaneous soft tissues.
Two cases of spontaneous pneumothorax in intranasal cocaine users have been reported from Italy [ ].
A 30-year-old man, a cocaine sniffer, who had used cocaine more than five times a month for 4 years, complained of shortness of breath and acute chest pain. He had episodic cough and bloody sputum. A chest X-ray showed an 80% pneumothorax on the left side. On thoracoscopy the entire lung visceral pleura seemed to be covered by fibrinous exudate. After yttrium aluminium garnet (YAG) laser pleurodesis surgery, which abrades the pleura, he made a full recovery within 4 days.
A 24-year-old man who had been inhaling cocaine nasally 4–5 times a month for a year developed respiratory distress and chest pain 2 days after the last use, because of a pneumothorax. He underwent video-assisted thoracoscopic surgery with laser pleurodesis and responded rapidly.
In both cases, pneumothorax occurred with a delay after cocaine inhalation. The authors suggested that it was therefore unlikely that these cases of pneumothorax were due to direct traumatic effects of the drug powder inhaled, to barotrauma due to exaggerated inspiration, or to a Valsalva maneuver. Histological examination in both cases showed small foreign body granulomas with polarized material in the subpleural parenchyma. The authors proposed that the pleural damage could have been directly caused by a filler substance known as mannite (a fine white powder comprised of insoluble cellulose fibers).
Spontaneous pneumomediastinum has been reported with the inhalation of free-base cocaine [ ].
Cocaine can cause exacerbation of asthma. All adult visits to an urban emergency room for an asthma attack during a 7-month period were reviewed [ ]. Of 163 patients (aged 18–55 years), 116 agreed to participate in a facilitated questionnaire and 103 provided urine samples for drug screening. African-Americans made up 89% of the group and 35% were cigarette smokers. Urine toxicology was positive for cocaine in 13% and for opiates in 5.8%. The severity of the exacerbation of asthma was greatest in the cocaine-positive group, 38% of whom were admitted to hospital (compared with 20% of the non-cocaine users). The length of stay was significantly longer in the cocaine-positive patients. Most of the patients did not use inhaled corticosteroids according to the treatment guidelines.
Empyema-like eosinophilic pleural effusion following the use of smoked crack cocaine has been reported [ ].
A 33-year-old man developed a fever of 104 °F, sweats, and a productive cough 1 week after using crack cocaine. He had diffuse wheezes at the lung apices and apical infiltrates and pleural effusions on CT scan. He had a raised white cell count at 18 × 109/l with 45% eosinophils. He was negative for acid-fast bacilli, HIV, and fungi. There was no history of travel or exposure to ill contacts or other medications. Pus-like pleural fluid 500 ml, drained from a left pneumothorax, was an exudate with large number of white cells with 80% eosinophils. Cultures of blood, sputum, bronchoalveolar lavage fluid, and pleural fluid were negative for bacteria and fungi. The bronchoalveolar lavage fluid contained many eosinophils and transbronchial biopsy showed an acute inflammatory infiltrate, with many eosinophils, edema, and no fibrosis, consistent with eosinophilic pneumonitis. The symptoms improved after chest drainage and glucocorticoid treatment. His illness resolved 1 month after discharge.
The authors speculated that a leak mediator, vascular endothelial growth factor, present in eosinophils, and cytokines implicated in the effects of cocaine and eosinophil activation may have contributed to the pleural effusion. They found the highest concentration of vascular endothelial growth factor ever reported at 20 ng/ml and increased pleural concentrations of IL-5, IL-6, and IL-8, suggesting potential roles for these cytokines in eosinophilic lung disease due to cocaine. They recommended that a pleural effusion that appears grossly to be pus in the setting of cocaine abuse should not be drained until an eosinophil predominant effusion is ruled out. If infection is excluded, an eosinophilic empyema in the setting of inhaled cocaine abuse should be treated with glucocorticoids and may not require drainage.
Inhalation of crack cocaine can mimic pulmonary embolism [ ].
A patient who was a known cocaine inhaler developed respiratory distress. The initial ventilation–perfusion lung scan was highly suggestive of pulmonary embolism, based on multiple segmental and subsegmental perfusion defects with normal ventilation. However, pulmonary angiography was normal. The symptoms resolved rapidly without anticoagulation and angiography was negative 2 weeks later.
The authors concluded that intense pulmonary artery vasospasm secondary to cocaine inhalation may have caused this syndrome.
Intranasal use, a common method of cocaine abuse, can damage the sinonasal tract, causing acute and chronic inflammation, necrosis, and osteocartilaginous erosion. These conditions occur secondary to the combined effects of direct trauma from instrumentation, vasoconstriction of small blood vessels with resultant ischemic necrosis, and chemical irritation from adulterants. Intranasal cocaine users can develop septal perforation, saddle-nose deformities, and sinonasal structural damage.
A 43-year-old woman with a past history of chronic heavy cocaine use and osteomyelitis of the hard palate and nasal cavity 10 years before had required continuous follow-up for recurrent ethmoid and sphenoid sinusitis [ ]. Endoscopy showed an absent nasal septum, middle turbinates, anterior two-thirds of the inferior turbinates, and lateral nasal wall.
Pott’s puffy tumor, a subperiosteal infection of the frontal bone, has been described in a 34-year-old man with a history of chronic intranasal cocaine use [ ].
This rare complication of frontal sinusitis appeared to develop secondary to the insertion of foreign bodies into the nose to facilitate inhalation of cocaine. Local trauma plus cocaine-induced vasoconstriction may have led to the complication.
Midline nasal and hard palate destruction have been reported in two chronic users of intranasal cocaine [ ]. The pathophysiology of these lesions is multifactorial, including ischemia secondary to vasoconstriction, chemical irritation from adulterants, impaired mucociliary transport, reduced immunity, and infection secondary to trauma.
In another case there was progression of septal perforation to secondary bone infection in a chronic cocaine user [ ].
A 56-year-old chronic intranasal cocaine abuser with a visible nasal defect presented with a hole in the roof of his mouth. He had been reportedly drug free for 2 weeks. He had an oronasal fistula with adjacent black necrotic areas and erosive destruction of the nasal septum, turbinates, and antrum, with mucoperiosteal thickening of the sphenoid and maxillary sinuses. Treatment included antibiotics and a prosthesis plate construction to cover the defect. Two years later, having continued to inhale cocaine, he had progressive destruction of his sinonasal tract, a fistula between his oral and nasal cavity, a saddle-nose deformity with total cartilage loss, and a complete palatal defect. Biopsy of the nasal septum showed acute osteomyelitis and extensive bacterial overgrowth (including anerobic Actinomyces -like organisms). He was given intravenous antibiotics for 6 weeks followed by long-term oral antibiotics.
Cocaine-related erosion of the external nasal structures has been described [ ].
A 43-year-old woman with a T12 paraplegia due to a car accident 24 years earlier and a sublabial abscess 2 years before developed progressive erosion of both the internal and external portions of her nose over 6 months, with nasal crusting and nose bleeds. Several antibiotics were unhelpful. There was partial destruction of the external nasal structure and two oronasal fistulae in the upper gingival sulcus. Intranasal biopsy showed acute and chronic inflammation. On two occasions urine drug screening was positive for cocaine, although she denied using cocaine.
Cocaine use can mimic vasculitis and is often accompanied by positive ANCAs. Cocaine-induced midline destructive lesions are characterized by mucosal damage and ischemic necrosis of the nasal septum. Histopathological similarity to leukocytoclastic vasculitis and the presence of PR3-ANCA can lead to confusion between Wegener’s granulomatosis and cocaine-induced midline destructive lesions.
A 30-year-old man developed destructive rhinitis due to cocaine abuse after initially presenting with Henoch–Schönlein purpura [ ].
Cocaine has been associated with necrosis of the nasal septum and necrosis of the hard palate [ ].
A combination of intranasal cocaine and paracetamol + oxycodone (Percocet) led to necrosis of the entire soft palate and most of the uvula in a 33-year-old woman, who developed oronasal reflux, voice changes, dysphagia, and partial hearing loss. Other causes of soft palate necrosis, including granulomatous disease and active infection, were ruled out. She underwent pharyngeal flap reconstruction, which addressed the velopharyngeal insufficiency, with improvement in speech intelligibility and reduced hypernasal speech.
The authors attributed this rare case of soft palate necrosis, without involvement of the hard palate, to inhalation of cocaine and Percocet.
From January 1991 to September 2001, 25 patients with cocaine-induced midline destructive lesions were observed at the Department of Otorhinolaryngology of the University of Brescia [ ]. There was septal perforation in all 25, and 16 also had partial destruction of the inferior turbinate. There was hard palate resorption in six patients and 14 were positive for antineutrophil cytoplasmic antibodies (ANCA). The need to consider substance abuse in the differential diagnosis of destructive lesions of the nasal cavity, even in the presence of antineutrophilic cytoplasmic antibodies, has been emphasized. There was constant progression of the ulceronecrotic process in 17 patients, and in three patients who stopped cocaine use the mucosa slowly normalized. Management consisted of periodic debridement of necrotic tissue and crusts, local and systemic antimicrobial drug therapy based on culture results, the use of saline douches for moistening the nasal mucosa, and surgical correction in severe cases.
All reports of cocaine-induced midline destructive lesions have been reviewed, and retrospective data involving 25 cases have been reported [ ]. All but three subjects admitted to cocaine abuse during the initial evaluation. There were 15 men and 10 women with a mean age of 36 years (range 22–66 years) and they had abused cocaine for 2–30 years, at a dose of 1–180 g/week. At rhinoscopy, all had necrotizing ulcerative lesions, extensive crusting, and septal perforation. The destructive process extended to the inferior (68%), middle (44%), and superior turbinates (16%). There were hard and/or soft palate perforations in six patients (24%); the lateral wall of the nose was entirely reabsorbed in five. The lesions caused both dysphagia and nasal reflux. In two subjects there was ulceration at the base of the columella at diagnosis. During follow up, two patients developed a nasocutaneous fistula. At diagnosis or during follow-up, none had any laboratory finding suggestive of a systemic disease, but 22 had nasal swabs positive for Staphylococcus aureus . Fungi were not grown. During the course of disease, two patients with severe diffuse destructive lesions had acute orbital symptoms and signs (diplopia, pain, and proptosis), caused by infection. One patient had a secretory otitis media. All had septal erosion. In 15 there was altered olfaction. The authors speculated that the possible mechanism included either direct damage to the neuroepithelium from cocaine or its adulterants or obstruction of the olfactory cleft by inflammation and edema of the nasal mucosa. Vascular abnormalities mimicking vasculitis were present in 23 subjects. The authors observed a constant progression of the ulcerative process in 17 patients. Three subjects who stopped using cocaine had slow normalization of the mucosa. The authors suggested that any sinonasal inflammatory condition involving the midline structures characterized by symptoms such as nasal obstruction and crusting that persists or remains refractory to treatment may be the first manifestation of a potentially lethal drug addiction.
Many surgeons in the UK use local anesthesia with cocaine for nasal operations because of the superior operative field it provides and because they consider it to be safe, even with adrenaline. The incidence of adverse reactions to cocaine given in this way is reportedly low, and serious complications are less common than with general anesthesia. These conclusions were based on a postal survey of all British Associations of Otolaryngologists and Head and Neck Surgeons. Only 11% of surgeons had experienced cocaine toxicity in their patients, and there had been only one recorded death [ ].
Research in 21 cocaine users and 13 non-drug-using, age-matched controls has suggested that chronic cocaine use may be associated with specific neurochemical changes in the brains of habitual users [ ]. All the subjects underwent a spectral brain scan in a proton MRS scanner. The significant finding was that the concentration of N-acetylaspartate in the left thalamus (but not in the basal ganglia) was significantly lower (17%) in the chronic cocaine users than in the controls. N-acetylaspartate is found in adult neurons and not in glia; it is often used as a marker of neuronal viability; a reduction suggests neuronal damage and/or loss.
Chronic substance use has been associated with long-lasting changes in brain function [ ]. Five brain regions that may be affected (the orbitofrontal gyrus, rectal gyrus, anterior cingulate gyrus, basal ganglia, and thalamus) were selected for analyses of cerebral glucose metabolism by positron emission scanning in controls, cocaine users, and alcoholics (17 in each group), who performed the Stroop test, which assesses cognitive interference and response inhibition. In controls, higher brain glucose metabolism in the orbitofrontal gyrus correlated with poorer performance. In contrast, in substance users, higher brain glucose metabolism was associated with better performance. Chronic abuse appears to be associated with altered function of the orbitofrontal gyrus.
Neuroimaging research has significantly improved our understanding of the neurobiological effects of acute cocaine on the brain. Brain regions such as the basal forebrain, caudate, nucleus accumbens, thalamus, ventral tegmentum, cingulate, and prefrontal cortex are activated during cocaine use. Electoencephalographic measurements have elucidated the acute effects of cocaine on brain neurophysiology. Quantitative electroencephalographic profiles were obtained from 13 crack cocaine-dependent users who self-administered single-blinded doses of smoked cocaine base 50 mg versus placebo in monitored sessions [ ]. Cocaine rapidly increased the absolute theta, alpha, and beta power of the prefrontal cortex for up to 25 minutes. Increased theta power was associated with reported “good drug effect” and increased alpha power with reported “nervousness”. Increased delta power was positively associated with plasma cortisol concentration and negatively with reported nervousness. Placebo increased alpha power in the prefrontal cortex. The authors propose that slow wave delta and theta activity are involved in the rewarding properties of cocaine.
Cocaine also affects cerebral blood flow. Hypoperfusion of the orbitofrontal cortex and possible differences among male and female cocaine users has been reported in 35 abstinent cocaine-dependent subjects and 37 healthy controls, using single photon emission computed tomography for regional cerebral blood flow after infusion of cocaine or saline [ ]. There were sex-specific anatomical differences: regional blood flow was reduced bilaterally in the orbitofrontal cortex in male cocaine users and in the medial orbitofrontal cortex in female cocaine users compared with controls. The authors explained that the orbitofrontal cortex is involved in assessing prospective rewards and making decisions. When it is injured, a person tends to make impulsive impaired decisions. They suggested that these sex differences may be important in developing methods for treating relapses.
Hypertensive encephalopathy can follow the use of cocaine [ ] and a fatal cocaine-related encephalopathy has been reported [ ].
A 21-year-old man with a history of depression and no prior cocaine use was found comatose in a hotel room with injection marks in the left cubital fossa. A syringe, empty packets with traces of white powder, later identified as cocaine, and a farewell letter were also found. He was unresponsive, with intact brain stem reflexes, bilateral hyper-reflexia, and moderately increased muscle tone. There was a mild metabolic acidosis (pH 7.30), raised liver enzymes, rhabdomyolysis, leukocytosis, and increased creatinine and hyperkalemia. There was cocaine in the urine. Electroencephalography after 3 and 12 days showed diffuse 2–4 Hz activity, with 2–30 second episodes of flattening and lack of response to visual or tactile stimulation. An MRI scan of the brain on day 21 showed diffuse symmetrical white matter lesions in the cerebrum, with preservation of U fibers. Proton nuclear magnetic resonance spectroscopy (1H-MRS) on day 21 in the occipitoparietal white matter and mid-occipital grey matter showed significant reductions in N-acetylaspartate, myoinositol, and creatine, with increased lactate, lipids, and macromolecules, consistent with major neuroaxonal injury and white matter demyelination. He died at 24 days. Autopsy showed severe leukoencephalopathy, with demyelination and liquefaction of the central cerebral white matter. The cortex, subcortical white matter and U fibers, brain stem, cerebellum, and hippocampus appeared relatively intact.
The authors pointed out that whereas the clinical presentation may vary, the radiological and histological findings in toxic leukoencephalopathy due to cocaine or other drugs of abuse are fairly characteristic.
Cocaine has been associated with movement disorders, such as acute dystonias, choreoathetosis, and akathisia. Chronic pancerebellar dysfunction occurred in a cocaine user with schizophrenia treated with risperidone [ ].
A 38-year-old man was found comatose in a crack house. The ambient temperature was 13 °C. He had earlier abused cocaine. His temperature was 43 °C, heart rate 115, blood pressure 144/89 mmHg, and oxygen saturation 97% on air. His general muscle tone was flaccid. He had a mild leukocytosis and hypophosphatemia. Urine toxicology was positive for benzoylecgonine, a cocaine metabolite. He was mechanically ventilated, cooled, and given intravenous fluids. His temperature fell to 38 °C, but he later developed acute disseminated intravascular coagulation and rhabdomyolysis. After 5 days he developed nystagmus, intention tremor, truncal ataxia, dysarthria, ocular dysmetria, and dysmetria of the arms and legs. There were no sensory or motor deficits. Finger-to-nose and heel-to-knee tests were slowed and uncoordinated. He could not stand. Brain imaging studies (CT and MRI scans) were unremarkable. He was given thiamine, propranolol, clonazepam, primidone, and baclofen every 8 hours without improvement. After 1 year he still had nystagmus, intention tremor, ataxic gait, and dysmetria. He could walk short distances slowly.
Cocaine and neuroleptic drugs can both cause hyperthermia and the authors proposed that the combination of cocaine and risperidone may have caused this problem.
About 60–75% of chronic cocaine abusers report severe headaches [ ], which can resemble migraine; migraine-like symptoms can include auras, visual field changes, and paraphasia [ ]. About 60–75% of chronic cocaine abusers report severe headaches [ ]. Of 21 patients who were admitted to hospital from January 1985 to December 1988 for acute headache associated with cocaine intoxication 15 had headaches with migrainous features in the absence of neurological or systemic complications [ ]. None had a history of cocaine-unrelated headaches or a family history of migraine, and all had a favorable outcome. The authors discussed three possible mechanisms of cocaine-related vascular headaches, depending on the interval between cocaine ingestion and development of the headache. They postulated that acute headaches after cocaine use may relate to the sympathomimetic or vasoconstrictive effects of cocaine, while headaches after cocaine withdrawal or exacerbated during a cocaine binge may relate to cocaine-induced effects on the serotoninergic system.
The effects of cocaine or its withdrawal on neurotransmitter activity have been evaluated in several studies. Although changes in dopaminergic activity appear to be associated with early cocaine abstinence, extrapyramidal symptoms (due to alterations in dopamine functioning) have only infrequently been reported in cocaine users. However, in a recent report, extrapyramidal symptoms of classic muscle stiffness and cogwheel rigidity at the elbow occurred during the “crash” phase of a 40-year-old man’s cocaine withdrawal [ ].
Cocaine can cause other movement disorders [ ]. Motor and vocal tics, pre-existing tremors, and generalized dystonia were induced or exacerbated by drug use, and continued even after cocaine use ended. Lastly, dopamine dysregulation (that is reduced dopamine receptor availability in association with reduced frontal metabolism) was demonstrated by positive emission tomography in 20 chronic cocaine abusers [ ]. The findings were prominent in the orbitofrontal, cingulate, and prefrontal cortex 3–4 months after detoxification. The author hypothesized that dysregulation of these brain areas may result in compulsive drug-taking behavior.
Dystonia has been described shortly after the use of cocaine [ ]. In a retrospective study of 116 patients taking neuroleptic drugs, 42% of cocaine users versus 14% of non-users developed dystonia [ ]. This suggests that the use of cocaine may be a major risk factor for acute dystonic reactions secondary to the use of neuroleptic drugs.
Cocaine-induced chronic tics have been reported [ ], and cocaine has reportedly exacerbated Gilles de la Tourette syndrome [ ].
Choreoathetoid movements have been evaluated in samples of cocaine-dependent men (n = 71), amphetamine-dependent men (n = 9), and 56 controls [ ]. The cocaine-dependent men had significantly worse non-facial (limbs plus body) choreoathetosis scores, and the differences between groups were most marked in the younger age groups. The facial scores were increased only in those under 32 years of age, as they were in the younger amphetamine-dependent subjects. The authors suggested that the absence of choreoathetoid movements in the older cocaine-dependent men may represent an age-related self-selection effect.
Alpha-synuclein is a presynaptic protein that has been implicated as a possible causative agent in the pathogenesis of Parkinson’s disease. In a study of postmortem neuropathological specimens from cocaine users (n = 21) and age-matched drug-free controls (n = 13), the concentrations of alpha-synuclein in dopamine-containing cells of the substantia nigra and ventral tegmental area were increased threefold in chronic cocaine users compared with controls with changes in the expression of alpha-synuclein mRNA [ ]. Although alpha-synuclein is prominent in the hippocampus, there was no increase in this area. Alpha-synuclein concentrations were increased in the ventral tegmental but not the substantia nigra area in victims of excited cocaine delirium who had paranoia, marked agitation, and hyperthermia before death. The authors speculated that increased alpha-synuclein may be a protective response to changes in dopamine and increased oxidative stress resulting from cocaine abuse. On the other hand, this accumulation of alpha-synuclein with long-term cocaine abuse may put addicts at increased risk of the motor abnormalities of Parkinson’s disease.
A 24-year-old woman developed myasthenia gravis during cocaine use [ ]. The authors speculated that while cocaine did not cause impairment of motor axons for neuromuscular transmission, a reduction in the number of acetylcholine receptors per neuromuscular junction (as occurs in myasthenia gravis) could have increased the susceptibility to an effect of cocaine.
A rare case of polysubstance abuse, which unmasked myasthenia and caused complete external ophthalmoplegia, has been reported [ ].
A 29-year-old woman, who used cocaine 2 g/day, heroin 1 g/day, and methadone 40 mg/day, developed abscesses caused by drug injection. She had had generalized weakness, difficulty in swallowing, and lagging eyelids for 1 week. There was bilateral ptosis, and a diagnosis of myasthenia was made. Edrophonium 10 mg relieved the ptosis and improved ocular movements.
Cocaine lowers the seizure threshold and may therefore be dangerous to patients already at risk of seizures [ ]. Exacerbation of generalized tonic-clonic seizures (which occurred initially during the use of crack but later continued independently of the drug) has been described, with progressive electroencephalographic abnormalities [ ]. In this case, the development of the seizures suggested that cocaine can stimulate kindling, with progressive intensification of after-discharges and the eventual emergence of seizure activity.
Cerebrovascular vasoconstriction and a sudden increase in blood pressure probably underlie the many reports of cocaine-induced strokes, CNS hemorrhage, and migraine [ ]. Three cases of generalized seizures occurred shortly after the intravenous use of cocaine [ ].
The disruption of normal sleeping patterns during cocaine withdrawal may be related to effects on the cholinergic system. In a study of nine patients undergoing cocaine withdrawal, rapid eye movement (REM) latency was markedly shortened, REM sleep percentage was increased, REM density was very high, and the total sleep period was long during the first week [ ]. Changes in REM sleep are thought to be related to changes in cholinergic activity. At week 3, characteristic chronic insomnia was observed.
There has been a longitudinal study of maternal substance use and child sleep behavior in 65 cocaine-exposed and 53 non-exposed infants and their primary care-givers, recruited at the time of delivery [ ]. By 7 months 18 of the cocaine-exposed infants had been removed from parental care, compared with only one of the non-cocaine group. At 7 months the care-givers’ psychopathology was assessed with the Brief Symptom Inventory (BSI) and the Maternal Cognitions about Infant Sleep questionnaire, a measure of mothers’ awareness about their infants sleep pattern, was administered. Infant physiological assessment during rest was done at both 4–8 weeks and 7 months. The severity of sleep difficulties significantly related to the severity of prenatal cocaine exposure, and maternal anxiety had a key role in this association. Altered sleep regulation in 7-month-old infants was strongly influenced by the care-givers’ environment. Non-maternal care-givers had fewer mental health problems than cocaine-using mothers. Infants who were in non-parental care had milder sleep problems than those who remained in parental care.
Dysregulation of sleep with impairment of cognition and learning associated with cocaine has been studied [ ]. In a 23-day in-patient study, 12 abstinent chronic cocaine users self-administered blinded dosages of either intravenous cocaine (8, 16, and 32 mg per 70 kg at 30 minute intervals) or placebo on each of study days 4, 5, 6 18, 19, and 20 in 2-hour sessions. Six took cocaine on days 4–6 (early bingers) and the other six on days 18–20 (late bingers). There was a marked discrepancy between recorded sleep and self-reports. Over the period of controlled abstinence (up to 17 days), sleep deterioration as measured objectively (total sleep time, sleep latency, and times awake after sleep onset) was timed to the sleep that chronic cocaine users reported as “at its best”. Spectral activity showed increased slow wave sleep, suggestive of chronic insomnia. Vigilance and procedural learning were also impaired. The authors suggested that chronic cocaine users are unaware of “occult insomnia”, which relates to dysregulation of homeostatic sleep drive. In this proposed mechanism, increased time awake is not recognized and translated into a drive to sleep.
Cocaine has been associated with cerebrovascular events, such as transient ischemic attacks [ ], cerebral hemorrhage [ , ], and cerebral infarction [ ].
Regional cerebral blood flow was assessed using single photon emission computed tomography (SPECT) and tracer HMPAO in 10 cocaine abusers within 72 hours of last cocaine use and then after 21 days of abstinence [ ]. Compared with controls, recent cocaine abusers had significantly reduced cerebral blood flow in 11 of 14 brain regions, with the largest reductions in the frontal cortex and parietal cortex and greater cerebral blood flow in the brain stem. These perfusion defects appeared to be primarily due to combined abuse of alcohol and cocaine. Frontal but not parietal defects appeared to resolve partially during 21 days of abstinence.
Cocaine has been associated with significant reductions in cerebral blood flow, thought to be secondary to its vasoconstrictor effects [ ]. In 13 chronic cocaine abusing men (mean age 38, range 28–45) and 10 healthy aged-matched male subjects, cerebrovascular pathology was assessed with functional magnetic resonance imaging (fMRI) to compare the abnormal blood oxygenation level dependent (BOLD) responses to photic visual stimulation. Cocaine abusers had a significantly enhanced positive BOLD response to photic stimulation compared with controls. The authors proposed that the enhanced activation in the cocaine abusers could have resulted from low resting cerebral blood flow secondary to increased vasoconstriction and/or from low oxidative metabolism during activation. Alternatively, the larger signal intensity in the cocaine abusers could have resulted from inefficient neuronal processing, as has been reported in other conditions of cerebral pathology.
In a review of ischemic stroke in young American adults (aged 15–44 years) admitted to 46 regional hospitals between 1988 and 1991, illicit drug use was noted in 12% and was the probable cause of stroke in 4.7% [ ]. Multidrug use was common among users: 73% used cocaine, 29% used heroin, and 14% used phencyclidine. Drug-associated stroke in these young adults appeared to be related to vascular mechanisms (such as large and small vessel occlusive disease) rather than to hypertension or to diabetes. Cerebral infarction is significantly more common among users of cocaine alkaloid (crack) than cocaine hydrochloride [ ]. Ischemic stroke has been attributed to combined use of cocaine and amphetamine [ ].
A previously healthy 16-year-old man developed an unsteady gait and double vision. His symptoms began 5 minutes after intranasal “amfetamine” (actually amfetamine cut with cocaine). He had a left-sided internuclear ophthalmoplegia, an incomplete fascicular paresis of the left oculomotor nerve, and saccadic vertical smooth pursuit. Cranial MRI showed a left-sided hyperintense lesion near the midline of the mesencephalon. A repeat MRI scan 9 days later showed that the lesion was much smaller. He made a full recovery within 3 weeks.
The cause of cocaine-related stroke and transient ischemic attacks has been studied by transcranial Doppler sonography, a continuous measure of cerebral blood flow velocity, to monitor the course of cerebral hemodynamic changes during acute intravenous injection of placebo, and of cocaine 10, 25, and 50 mg in seven cocaine abusers [ ]. There was a significant increase in mean and systolic velocity (lasting about 2 minutes) with all doses of cocaine but not with placebo. Cocaine produced an immediate brief period of vasoconstriction (as demonstrated by an increase in systolic velocity) in the large arteries of the brain.
A bilateral hippocampal stroke, which is rare, has been attributed to cocaine [ ].
An unresponsive 25-year-old white woman was found to be pulseless and was resuscitated. She had used cocaine the previous night and had had numerous psychiatric admissions for mood disorder and drug overdoses in the past. Her pupils measured 4 mm bilaterally and were non-responsive to light. She had roving eye movements and positive corneal responses. She did not respond to verbal or noxious stimuli. Her reflexes were brisk throughout, with clonus in the legs. There were cocaine metabolites in her urine. CT and MRI scans of the brain showed bilateral hippocampal and basal ganglionic hypodensities. She improved and was discharged to a nursing home 3 weeks later but continued to have severe short-term memory difficulties, problems with praxis, and mild quadrispasticity.
The authors cited a combination of ischemia and excitotoxicity due to cocaine exposure as the possible cause of the brain injury. Oxidative stress and free radicals associated with cerebral hypoxia contribute to cell damage and death.
Analysis of intracranial hematomas for detecting drugs with short half-lives in individuals who survive several hours after intracranial bleeding has been recommended for establishing the cause of death. Cocaine metabolites were found in an intracerebral hematoma in a patient who apparently died due to cocaine [ ]. The hematoma contained ethanol 0.05% w/v and benzoylecgonine 0.43 mg/l.
“Spontaneous” acute subdural hematoma related to cocaine abuse has been described [ ].
A 38-year-old man with a 10-year history of cocaine use became comatose. He had had an acute severe headache and progressive deterioration after abusing cocaine. His Glasgow Coma Scale was 3 and his pupils were dilated but reactive to light. He had hypertension and bradycardia. Routine toxicology was positive for cocaine. Blood tests, including coagulation profile, were normal. A CT scan of the brain showed a left acute subdural hematoma with midline shift and obliteration of the basal cisterns. During emergency craniotomy the source of the bleed was identified as a pinhole rupture of a parietal cortical artery. The patient had no history of head injury and there were no intraoperative findings of head injury. He died 24 hours later without evidence of clot reaccumulation. An autopsy was not performed.
Brown–Séquard syndrome after esophageal sclerotherapy and recent crack cocaine abuse has been reported [ ].
A 44-year-old man with hepatitis C and cirrhosis, esophageal varices, and poorly controlled hypertension, who was also a chronic alcoholic and crack cocaine abuser, had his varices injected at endoscopy and developed weakness and numbness up to T4. There was flaccid right leg weakness, right T4-6 hypalgesia, left leg hypalgesia up to L1, and reduced sweating on the left up to T4-6. An MRI scan of the thoracic spine showed a lesion at T4-6 involving the anterior and central portions of the spinal cord. A urine screen was positive for cocaine. He recovered spontaneously 2 weeks later.
Cocaine-induced ischemia was the most likely cause of this adverse outcome.
There is an association between cocaine use and aneurysmal subarachnoid hemorrhage [ , ]. Subarachnoid hemorrhage was temporally related to cocaine abuse in 12 young adult abusers who had underlying cerebral aneurysms; hypertension was a probable contributing factor [ ] and cocaine is a risk factor for cerebral vasospasm after aneurysmal subarachnoid hemorrhage [ ]. In a retrospective analysis of the medical records of 440 patients who presented to a neurosurgery unit between 1992 and 1999 with aneurysmal subarachnoid hemorrhage, 27 patients (6.1%) had either a positive urine screen for cocaine metabolites (n = 20) or a history of cocaine use within 72 hours of subarachnoid hemorrhage (n = 7). Cocaine users were more likely to have cerebral vasospasm from 3 to 16 days after subarachnoid hemorrhage than non-exposed patients (63 versus 30%). They were also more likely to be younger and to have aneurysms of the anterior circulation than the control group (97 versus 84%).
The adverse effect of cocaine on the clinical course in subarachnoid hemorrhage has been studied in a retrospective review of the medical records of 151 patients with intracranial aneurysms treated at a Taiwanese hospital between January 1996 and December 2001 [ ]. Of 108 patients who had subarachnoid hemorrhage, 36 had used cocaine within the previous 24 hours and 20 of them had subarachnoid hemorrhages of greater severity (Hunt and Hess grade of IV or V) compared with eight of 72 non-cocaine users. There was significant angiographically confirmed vasospasm in 28 of the cocaine users and 20 of the non-users. There was a 2.8-fold greater risk of vasospasm associated with cocaine use. Cocaine has vasoactive properties that both influence and increase the occurrence of cerebral vasospasm, the main cause of morbidity and mortality in patients with subarachnoid hemorrhage who survive the initial event.
Ophthalmic effects associated with cocaine can occur during both active drug use and early abstinence. Cocaine abuse has been associated with ophthalmic complications, including ulceration of the cornea, vasoconstrictor effects on the retinal vasculature, irregularities in oculomotor performance, and secondary optic neuropathy.
Corneal disturbances have been reported with crack cocaine, including ulcerative keratitis [ ].
A 42-year-old African–American woman developed blurred vision and a mucopurulent discharge and progressive pain in her left eye. She had a history of polysubstance abuse, but no history of contact lens use, trauma, or surgery. She had injected heroin the evening before and again several hours later. The left cornea had a deep paracentral stromal ulcer with an overlying epithelial defect. Toxicology screen was positive for cocaine and heroin. Corneal cultures grew Candida albicans . With topical therapy the lesion regressed in size and her symptoms abated.
Ulcerative keratitis is a sight-threatening condition. Crack-cocaine vapors can have a direct toxic effect, which impairs the protective capacity of the corneal epithelium and predisposes the corneal tissue to blemishes and infection. They also have anesthetic properties, which may increase the risk of mechanical trauma from excessive eye rubbing.
Corneal ulceration secondary to smoking crack cocaine has been reported in a 27-year-old woman [ ].
Cases of the “crack eye syndrome” continue to be reported. Of 14 crack cocaine users with corneal problems, 10 had corneal ulcers infected with both bacterial and fungal organisms; 4 had corneal epithelial defects [ ]. All were actively smoking crack daily. The authors suggested that crack smoking predisposes users, through an unknown mechanism, to corneal epithelial changes, infection, and perforation. Typical presentations include loss of vision with or without pain.
A 29-year-old woman with a painful corneal ulcer related to cocaine abuse was found to be putting cocaine powder directly into the affected eye to reduce the pain [ ]. Her history included prior corneal perforations.
Such topical use of cocaine may aggravate the condition.
Acute iritis has been reported after intranasal use [ ].
There has been a report of retinal changes in 60 users of crack [ ]. Microtalc retinopathy and retinal nerve fiber layer “rake” or “slit” defects were detected by threshold visual field testing and fundus photography.
Orbital infarction has been described after cocaine use [ ].
A 36-year-old woman drank alcohol and snorted cocaine and heroin at a party. She lost consciousness, with her head positioned down with her left face pressed against a desk. She awoke 3 hours later with severe left orbital pain. Her right eye was normal but there was complete visual loss in the left eye and a nearly complete left ptosis. The left pupil did not react to light but reacted consensually. Movements in the right eye were full, but movements in the left eye were severely limited in all directions. In the left fundus there was retinal edema and retinal pigment epithelium disruption. An orbital MRI scan showed diffuse swelling of all the extraocular muscles in the left orbit. A week later the pain had abated and there was mild improvement in the eye movements and ptosis, but no change in vision. She was instructed to wear protective polycarbonate lenses at all times.
Central retinal artery occlusion has previously been reported in cases of intravenous and intranasal cocaine abuse and has also been reported in a man who smoked crack cocaine [ ].
A 42-year-old man smoked crack and developed sudden painless loss of vision in his right eye for 9 hours. He had smoked cigarettes for 20 years and crack cocaine twice a week for the previous 4 years. Visual acuity in the right eye was counting fingers at one meter, and in the left eye 6/4. There was a right relative afferent papillary defect. In the right fundus there was evidence of central retinal artery occlusion and the left fundus was unremarkable. Treatment included intravenous acetazolamide, intermittent ocular massage, and rebreathing into a paper bag. He was found to have sickle cell trait, a risk factor for central retinal artery occlusion.
Pre-retinal hemorrhage has been attributed to use of cocaine.
A healthy 25-year-old man developed painless loss of vision in his right eye after using cocaine the night before [ ]. His visual acuity was 1.0 in his left eye and 0.05 in his right eye. There was pre-retinal hemorrhage in the right eye. Visual acuity did not improve over the next 2 weeks and the macular hemorrhage continued to spread. He therefore underwent pars plana vitrectomy. The visual acuity in his right eye improved to 0.9.
Assessment of smooth pursuit eye movements has been used in the study of neurophysiological effects of a variety of clinical and subclinical disorders. In 126 patients who met DSM-IIIR criteria for dependence on alcohol, cocaine, or heroin, or dual alcohol and cocaine abuse, there was a significant reduction in tracking accuracy in the heroin-dependent and the dually-dependent subjects relative to controls [ ]. However, eye movement dysfunction in the drug-dependent groups was not detectable when the effects of antisocial personality disorder were statistically removed. The magnitude of the dysfunction correlated significantly with several antisocial personality-related features, including an increased number of criminal charges, months of incarceration, increased problems associated with drug abuse, and lower intellectual functioning. The findings suggested that there may be an association between premorbid personality traits and eye movement impairment.
Cocaine use has been associated with a reduced inhibitory response of the P50 auditory evoked response, attributed to increased catecholaminergic and reduced cholinergic neurotransmission secondary to cocaine [ ]. In a double-blind, placebo-controlled study, 11 cocaine users in the first and third weeks of detoxification had electrophysiological testing 10 minutes before and 30 minutes after taking nicotine gum 6 mg. Nicotine briefly reversed the inhibitory deficit.
Cochleovestibular deficit has been attributed to intralabyrinthine hemorrhage after cocaine consumption [ ].
A 43-year-old man taking maintenance methadone used intranasal cocaine and drank alcohol and suddenly developed tinnitus in the left ear and progressive giddiness. He had left deafness, right spontaneous horizontal nystagmus, and left areflexia on caloric stimulation. A diagnosis of cochleovestibular deficit was made. A week later the right spontaneous horizontal nystagmus had resolved but was elicitable by a head-shaking maneuver. An MRI scan showed blood in the left labyrinth. Neurological and ophthalmological examinations were normal. The giddiness resolved after several weeks but his hearing did not recover.
The authors presumed that this disorder was due to cocaine-induced vascular effects. As cocaine prevents reuptake of amines by presynaptic receptors, increased concentrations of amines may lead to abruptly altered circulation.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here