Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
This chapter combines the traditional anatomical approach to embryology with the rapidly advancing understanding of developmental biological processes involved in eye formation and maturation. Knowledge of the gene networks involved in ocular development provides a foundation for understanding the impact of developmental disorders on visual function. Appreciation of ocular developmental gene networks is also critical for processes of induced pluripotent stem cell (iPSC) differentiation to ocular organoid model systems.
Developmental biology addresses the processes through which a single cell gives rise to a complex multicellular organism. These processes include mechanisms controlling the changes in shape (morphogenesis), cell type diversity (histogenesis), and functional maturation during embryogenesis.
Differentiation is the process whereby collections of cells develop into a multicellular organism with tissues of particular functions. Multicellular organisms are made of 3D spatiotemporal arrays of different cell types that develop over time. The fate map defines the individual intrinsic (gene) steps to achieve this. Increasingly recognized are the environmental factors (pH, metabolites, morphogens).
Collective cell migration plays essential roles in embryogenesis to facilitate the dramatic shape changes that occur over short periods of time. Differential rates of cell division within and between tissues contribute to these shape changes. Regulatory processes include self-generating gradients, internal chemotaxis or mechanotaxis and contact-dependent polarization within migrating cell groups. Neural crest cells (NCC) are one of the most striking examples of cell migration moving from their birthplace to a distant region including the developing eye.
Development includes processes of construction as well as remodeling (destruction). Apoptosis is critical for tissue remodeling enabling normal development.
Embryonic development depends on accurate, timely, and specific communication between cells. Cell communication involves several components, including ligands which are molecules that bind another specific target molecule, termed a receptor. Receptors can be on the cell surface or intracellular. The ligand–receptor interaction will often lead to a signal transduction cascade. Some ligand–receptor interactions can activate several different signal transduction pathways. By convention, the most commonly used pathway is known as canonical (e.g. β-catenin activation in Wnt signaling) and the alternative pathway is known as the non-canonical pathway. Highly conserved core pathways, including the Notch, Transforming Growth Factor beta (TGF-β), Wnt, Hedgehog (HH), and Receptor Tyrosine Kinase (RTK) signaling systems, play pivotal roles across a broad range of developmental processes.
Transcription factors (TFs) are proteins that bind DNA by recognizing specific sequence motifs located at regulatory elements, such as promoters and enhancers. In turn, this TF binding controls downstream processes such as recruitment of RNA polymerases, DNA methylation, and nucleosome chemical modifications and displacement. The result is the activation or repression of gene expression. The expression of combinations of TFs can be used to mark a region of undifferentiated tissue that will become a specific structure. RAX is an example that defines the eye field.
The vertebrate eye is formed through coordinated interactions between neuroepithelium, surface ectoderm, and extraocular mesenchyme. Three main periods are distinguished in prenatal ocular development. The first period (embryogenesis) involves the establishment of the primary organ rudiments and finishes when the optic groove (optic sulcus), the anlage of the eye, appears on either side of the midline at the expanded cranial end of the open neural folds around the end of the third gestational week. The second period (organogenesis) includes the development of the primary organ rudiments and extends to the end of the eighth week. The third period (differentiation) involves each of the primitive organs developing into a fully or partially active organ. This period starts at the beginning of the third month. During this period, the retina, optic nerve, vitreous, lens, and angle structures develop. A number of transcription factors and pathways have been identified in association with these developmental events through primarily model system studies (see Table 2.1 ).
| Gestational age | Developmental milestone, human | Transcription factors | Signaling pathways |
|---|---|---|---|
| <22 days | Eye field specification | RAX , OTX2 , Pax6 , Lhx2 , Six3 , Tbx3 , NR2E1 , Six6 |
|
| <22 days | Eye field splitting | Shh , Six3 | HH |
| 22 days | Optic vesical evagination | Rax , Pax2 , Pax6 , Tll | TGF (Transforming Growth Factor) |
| 28 | Lens placode formation | Pax6 | BMP, TGFβ/Smad3, Wnt/beta-catenin |
| 28 | Optic stalk differentiation | Shh , Pax2, Vax1, Vax2 | HH |
| 32 | Optic vesical invagination | Shh , Lhx2 , Bmp4 | HH, BMP, Retinoic acid (RA) |
| 32 | Lens placode invagination | Pax6 , Sox2 , Sox1 , Foxe3 , Six3 , Maf , Prox1 | TGFβ/Smad3, Wnt/beta-catenin |
| 33 | Optic cup patterning | Lhx2 , Pax6 , Pax2 , Mitf , and Vsx2 | TGF, SHH, WNT, BMP, RA |
| 33 | Start retinal neurogenesis in optic cup |
|
|
| Retinal pigment epithelium development | Pax6, Mitf, Otx2 | FGF, TGFβ, WNT, BMP, Notch and Hippo | |
| Sensory neural retina development | Otx2, Crx, Nrl, Nr2e3, Atoh7 | HH | |
| 42 | Hyaloid artery fills embryonic fissure | EphrinB2/STAT1/JNK3 | |
| 42 | Closure of embryonic fissure begins | Pax2 , Pax6 , Vax1 , Vax2 , Tbx2 , Chd7 , Bcl6 , Ephrin , Jnk , Tbx3 , Tbx5 , Vsx2 , Mitf , Foxc1 , Pitx2 | Wnt, FGF, RA, Hippo, Shh, BMP, TGFB |
| Lid folds appear | Fgfr2 , Erbb2 , Shh , Ptch1 and 2 , Smo , Gli2 , Jag1 Notch1 | EGF, Shh, Notch, BMP | |
| 49 | Neural crest cells (corneal endothelium) migrate centrally; corneal stroma follows | Pax6 , Pitx2 , Foxc2 , Lmx1b , CXCL14 | Retinoic acid (RA), |
| 49 | Choroidal vasculature starts to develop | Laminin, vitronectin | |
| 56 | Axons from ganglion cells migrate to optic nerve | Pax2 , Slit2 , CSPGs , Shh , Netrin-1 | Neutrins, semaphorins, laminin, Ephrin/Eph families |
| 56 | Lid folds meet and fuse | Fgfr2 , Erbb2 , Shh , Ptch1 and 2 , Smo , Gli2 , Jag1 , Notch1 | SHH, Notch, BMP, RA-RXR/RAR, MAP3K1-JNK, EGFR, ROCK, PITX2-DKK2 |
| 3rd month | Sclera condenses | Indian hedgehog (Ihh) | |
| 4th month | Retinal vessels grow into nerve fiber layer near optic disc | VEGF , bFGF , PDGF , Hif2a , LRP1 , FZD4 | VEGF, EphrinB2/STAT1/JNK3, SFRP |
| Schlemm’s canal appears | Angpt1 , Angpt2 , TEK | VEGF/VEGFR, ANGPT/TEK pathways | |
| Glands and cilia develop in lids | FGF10 , FGF7 | FGF, BMP, Wnt, Notch, Sox | |
| 5th month | Photoreceptors develop inner segments |
|
|
| Lids begin to separate | BMP, Smad | ||
| 6th month | Dilator muscle of iris forms | Pax6 | |
| 7th month | Central fovea thins |
|
|
| Fibrous lamina cribrosa forms | NRG1/ErbB4 | ||
| Choroidal melanocytes produce pigment | Tyr, Mitf | ||
| 8th month | Iris sphincter develops | Pax6 | |
| Chamber angle completes formation | FOXC1, FOXC2 | ||
| Hyaloid vessels regress | JNK3 , p-EphrinB/SHP2 complex | p-STAT1, p-EphrinB | |
| Retinal vessels reach periphery | VEGF | ||
| Optic nerve myelination complete to lamina cribrosa | Olig1 , Olig2 | Arp2/3 | |
| Pupillary membrane disappears | JNK3 , p-EphrinB/SHP2 complex | p-STAT1, p-EphrinB |
Early vertebrate eye formation follows a conserved sequence of events. Following gastrulation, the eye field is specified in the anterior neural plate extending across the midline. Studies in vertebrate model systems indicate that eye specification is defined by discrete and overlapping expression domains of different transcription factors (TFs) (see Table 2.1 ). The eye field transcription factors (EFTF) include: Otx2 , Tbx3 , Rax , Pax6 , Six3 , Six6 , Lhx2 , and Nr2e1 . Rax is the EFTF most used as a marker of the eye field ( Fig. 2.1 ). Mutations in the human Rax gene are a rare cause of human microphthalmia. Otx2 is the earliest molecular delineator of the eye field and mutations in Otx2 cause severe ocular malformations in humans. The eye field must split in the midline to form two separate eyes. Both Shh and Six3 are critical regulators of eye field bifurcation. Following splitting of the eye field, the first morphological landmarks are bilateral indentations (optic sulci or pits) in the cranial end of embryonic neural folds at approximately 22 days.
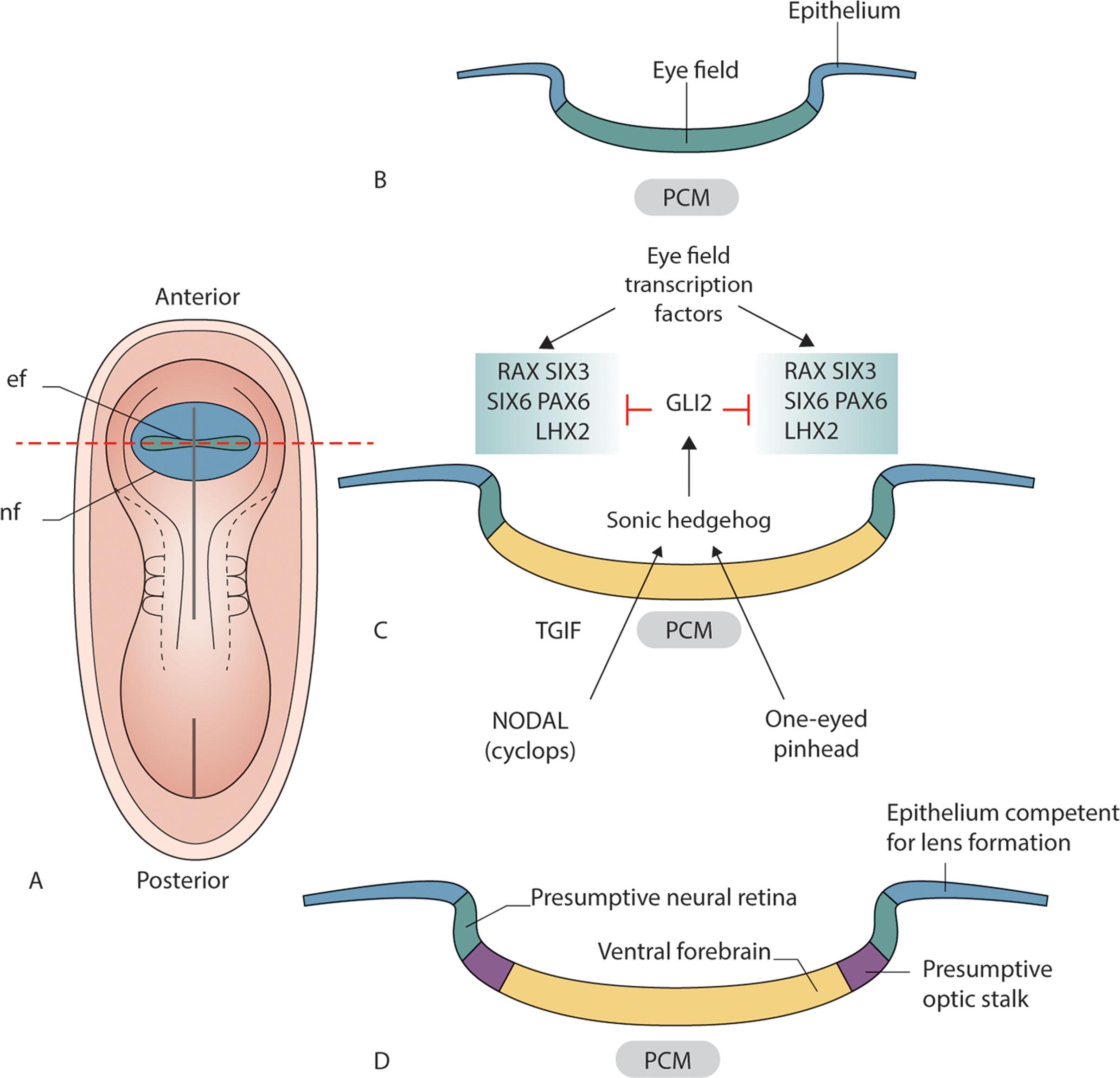
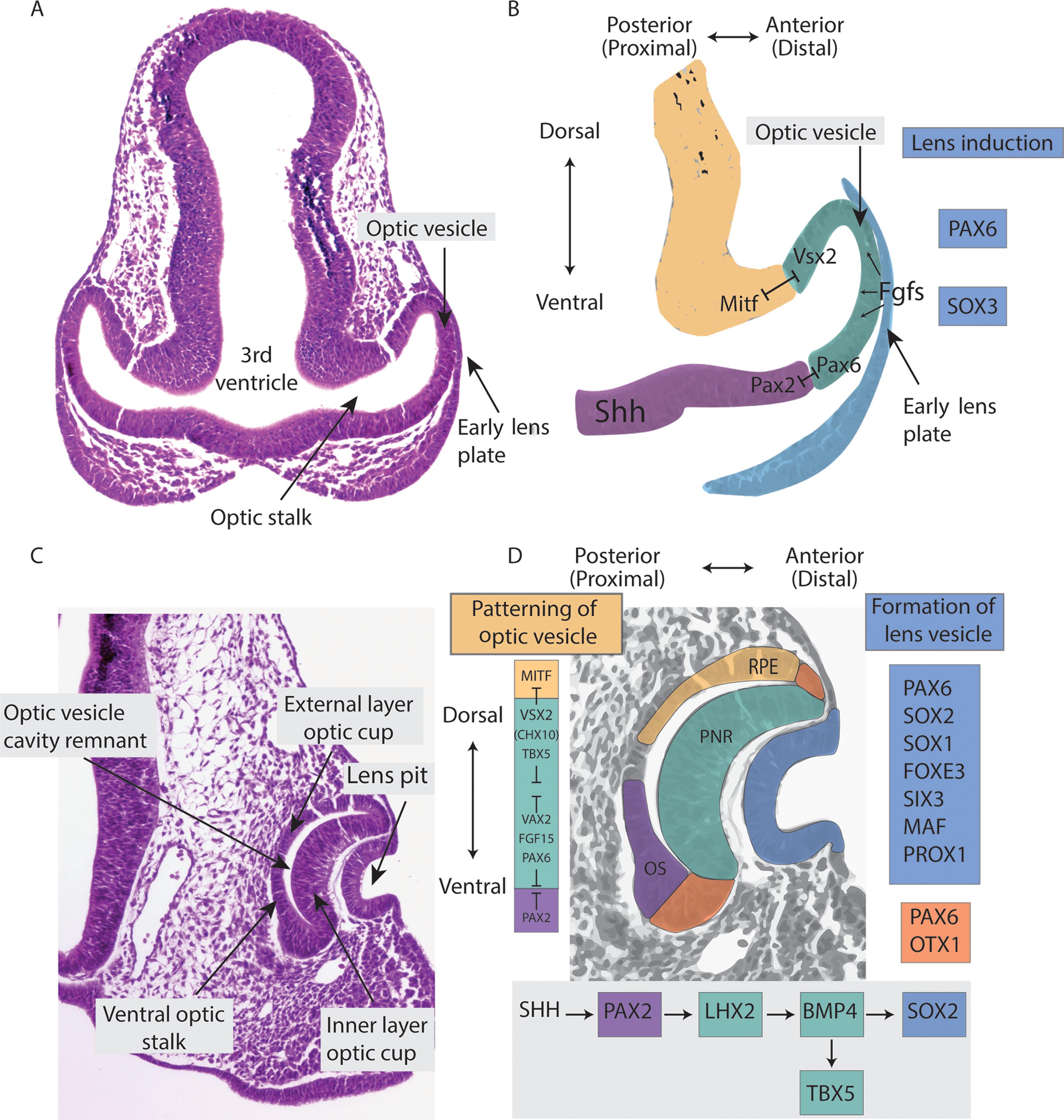
Following the establishment of the two eye primordia, the optic vesicle (OV) precursor cells continue migrating, initiating vesicle evagination. A subset of eye field transcription factors, including Rax and Pax6 , control OV evagination and formation (see Fig. 2.1 ). The OVs are connected to the forebrain by the optic stalk (a short tube that eventually forms the optic nerve) ( Fig. 2.2A–B ).
Organogenesis requires precise interactions between a developing tissue and its environment. In vertebrates, the developing eye is surrounded by a complex extracellular matrix as well as multiple mesenchymal cell populations. Disruptions to either the matrix or periocular mesenchyme can cause defects in early eye development.
Following evagination of the OVs, interaction between the OVs and surface ectoderm (SE) induces the lens placodes, and the wall of the OV in contact with the surface ectoderm also thickens to form the retinal disc ( Fig. 2.2C–D ). Towards the end of the 4th week a process of invagination begins to transform the OV into the optic cup (OC). Concurrently, the primordia of the extraocular muscles appear as condensations in the periocular mesenchyme.
The midline gradient of Shh signaling also differentiates the optic stalk (OS) from the optic cup. Shh induces expression of the optic stalk markers, Pax2, Vax1, and Vax2 ( Fig. 2.2D ). Reciprocal transcriptional repression between Pax2 and Pax6 creates a boundary demarcating the future optic stalk region and the presumptive neural retina. Pax2 expression is a marker of the presumptive optic stalk and Pax6 a marker of the presumptive neural retina ( Fig. 2.2D ). The transcription factors Foxc1 and Pitx2 in the periocular mesenchyme (POM) also play key roles in OC morphogenesis.
Disruptions in these early steps lead to severe congenital anomalies, including absent eyes (anophthalmia), small eyes (microphthalmia), and optic fissure closure defects (coloboma).
Further invagination of the OV to form the OC predominates in the 5th week. Invagination involves the retinal disc, lens plate, and the ventrocaudal wall of the optic vesicle ( Fig. 2.2C–D ). A critical developmental step is the patterning of the dividing OV progenitor cells to establish dorsal–ventral, nasal–temporal, and distal–proximal identity. The establishment of these molecular boundaries directs the OC to regionalize along several axes: dorsal–ventral, anterior (cornea)–posterior (retinal pigment epithelium, RPE), OS (ventral) nasal–temporal, and central (central neural retina)–peripheral (ciliary epithelium). Shh, retinoic acid (RA), and bone morphogenetic protein 4 (BMP4) are the key factors in patterning the developing OV. Bmp4 is expressed in the distal OV and subsequently within dorsal regions of the OC. The midline gradient of Shh signaling differentiates the OS from the OC through induction of the OS markers, Pax2, Vax1, and Vax2 ( Fig. 2.2D ).
Networks of transcription factors act by induction and repression to form the boundaries between the OS, RPE, and the presumptive neural retina (PNR). Key transcription factors include Pax2 , Pax6 , Vax1 , Vax2 , Tbx2 , Tbx3 , Tbx5 , Vsx2 , and Mitf . The establishment of these molecular boundaries is vital for subsequent steps in eye formation, as different regions of the OV give rise to the RPE (dorsal), OS (ventral), and PNR ( Fig. 2.2D ).
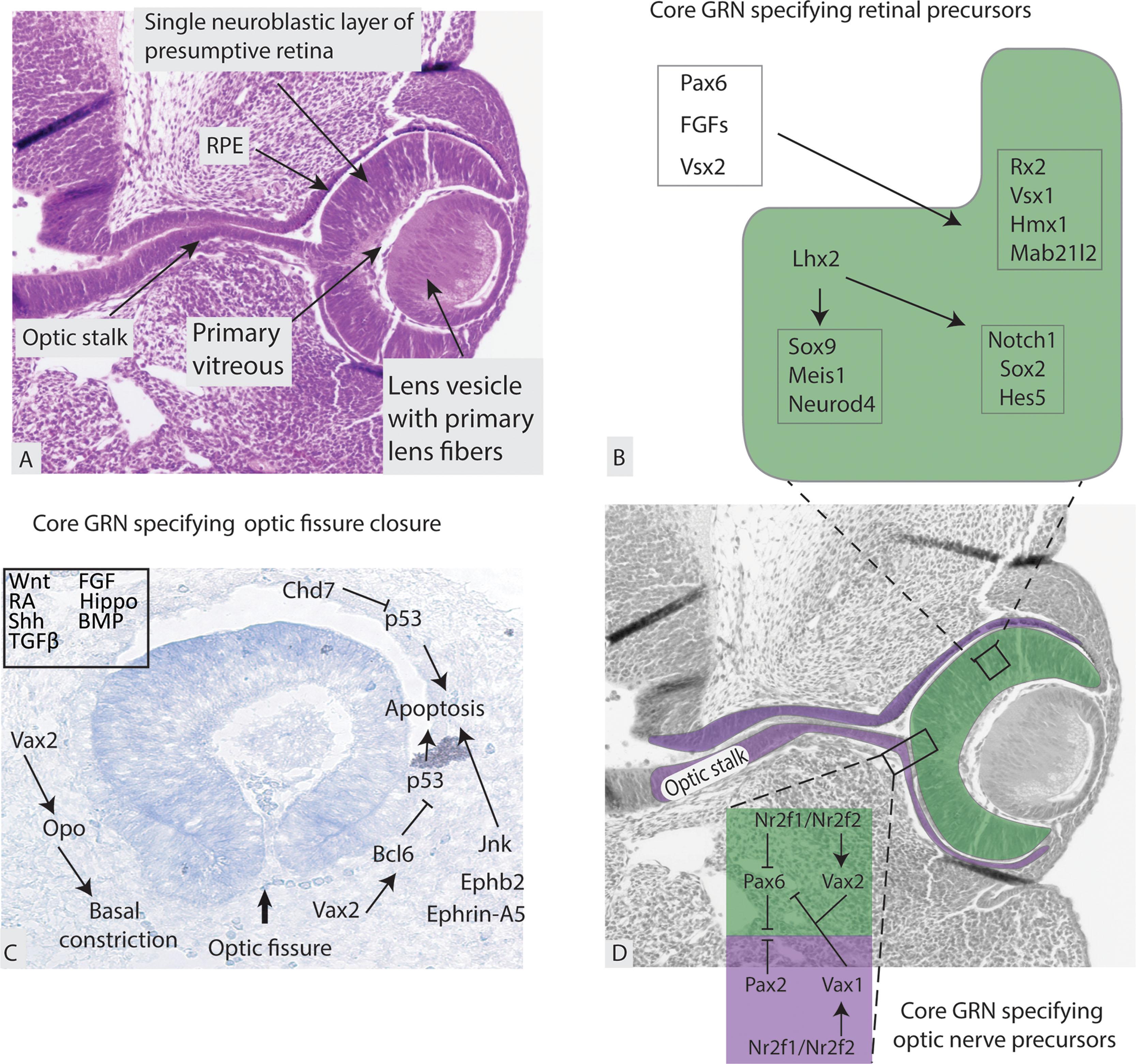
Invagination of the retinal disc of the OV leads to formation of the inner layer of the OC which becomes the PNR, while the external layer of the OC is the forerunner of the RPE ( Fig. 2.2D ). The OC is not continuous and forms a fold inferiorly and ventrally that is continuous with the OS. This fold is called the embryonic (optic) fissure.
Lens development from the lens placode is controlled by signals emanating from both the OV (positive) and periocular mesenchyme (POM) (negative). This occurs along the head surface ectoderm through Pax6 inhibition by TGFβ/Smad3 and Wnt/beta-catenin signaling from the POM to the ectoderm.
The lens pit deepens to become the lens vesicle. This occurs synchronously with optic cup invagination ( Fig. 2.2C–D ). The lens placode cells constrict apically leading to lens vesicle separation from the surface ectoderm which will become the corneal epithelium. The resulting lens vesicle is relatively large and fills the OC ( Fig. 2.3 ). Two key regulators of lens development are Pitx3 and Foxe3 . Pitx3 expression in the lens placode is more refined than Foxe3 but becomes expressed throughout the lens vesicle and is another key regulator of lens development.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here