Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
When it comes to direct, integrated, daily management of cardiac and pulmonary physiology, no specialty compares with anesthesiology. A sound understanding of the separate and integrated fundamentals of cardiorespiratory physiology is essential for an anesthesia provider to rapidly manage both critical and common situations such as hypotension, arterial hypoxemia, hypercapnia, and high peak airway pressures. The knowledge and skills required to excel as anesthesia providers provide invaluable insight when facing novel physiologic and logistic conundrums, as evidenced by anesthesiologists during the COVID-19 pandemic. This chapter reviews the basic tools and approaches every anesthesia provider must have in their armamentarium in order to provide safe, physiologically sound patient care. Although concepts are explained in discrete parts, the skilled anesthesia provider must also consider the integration and interdependence of multiple physiologic systems.
Systemic arterial blood pressure and mean arterial pressure (MAP) are commonly monitored by anesthesia providers via a blood pressure cuff or an indwelling arterial cannula. Although treatment of chronic systemic hypertension is sometimes necessary, acute hypotension is more often encountered during anesthesia care. Hypotension varies from mild, clinically insignificant reductions in MAP from general anesthesia or regional anesthesia to life-threatening emergencies. Hypotension can be of sufficient magnitude to jeopardize organ perfusion, causing injury and an adverse outcome. Organs of most immediate concern are the heart and brain, followed by the kidneys, liver, and lungs. All have typical injury patterns associated with prolonged shock. Understanding the physiology behind hypotension is critical for diagnosis and treatment.
Intraoperative hemodynamic instability has long been thought to result in worse outcomes after surgery. In large retrospective studies intraoperative hypotension of even 5 minutes' duration (systolic blood pressure [SBP] <70 mm Hg, MAP <50 mm Hg, diastolic blood pressure [DBP] <30 mm Hg) is associated with increased postoperative morbidity and mortality risks. , In a large 2020 observational study of patients undergoing noncardiac surgery intraoperative hypotension was associated with acute kidney injury in those patients in higher-risk groups (based on American Society of Anesthesiologists [ASA] physical status and type of surgery). Postoperative hypotension is also a risk factor for myocardial injury after noncardiac surgery.
This chapter will focus mainly on the physiology of the left ventricle (LV) in the discussion of hypotension. However, the contribution of the right ventricle (RV) and venous system is also important. To assess and treat acute hypotension, it is logical to start with the MAP and to consider it as the product of its physiologic components:
where SVR is the systemic vascular resistance and CO is cardiac output. When faced with an inadequate MAP, one should consider which component is the primary culprit. Additionally, although the clinician's focus tends to be on MAP alone, the contributing pressures (e.g., SBP, DBP, and pulse pressure [PP = SBP − DBP]) also require attention and can provide invaluable information when formulating a differential diagnosis and treatment plan. The pulse pressure is created by the addition of stroke volume (SV) on top of the DBP within the compliant vascular tree. The aorta is responsible for most of this compliance. Increased pulse pressure can occur with an increased SV, but most often occurs because of the poor aortic compliance that accompanies aging (also see Chapter 35 ). Decreasing DBP can have more dramatic effects on SBP when vascular compliance is poor.
Most drugs administered during general anesthesia and neuraxial regional anesthesia (also see Chapter 17 ) decrease SVR. Several pathologic conditions can produce profound reductions in SVR, including sepsis, anaphylaxis, spinal shock, and reperfusion of ischemic organs. The calculation for SVR is as follows:
where CVP is the central venous pressure, and the factor 80 converts units into dyne/s/cm 5 from pressure in millimeters of mercury (mm Hg) and CO given in liters per minute (L/min).
Pulmonary artery (PA) catheterization can be used to obtain the measurements necessary for calculating SVR, but this monitor is not usually immediately available. Signs of adequate perfusion (e.g., warm extremities, good pulse oximeter plethysmograph waveform and perfusion index) may sometimes be present when hypotension is caused by low SVR. On the other hand, hypertension nearly always involves excessive vasoconstriction. 1
1 Perfusion index is a measure of the pulsatile signal relative to the background absorption and is an important measure of signal strength.
According to Poiseuille's law, resistance is inversely proportional to the fourth power of the radius of a vessel. Individually, small vessels offer a very high resistance to flow. However, total SVR is decreased when there are many vessels arranged in parallel. Capillaries, despite being the smallest blood vessels, are not responsible for most of the SVR because there are so many in parallel. Most of the resistance to blood flow on the arterial side of the circulation is in the arterioles.
As a cause of hypotension, decreased CO may be more difficult to treat than decreased SVR. Increased CO is not usually associated with systemic hypertension, and most hyperdynamic states, such as sepsis and liver failure, are associated with decreased systemic blood pressure, which may lead to falsely believing the issue is low SVR alone.
CO is defined as the amount of blood (in liters) pumped by the heart in 1 minute. Although the amount of blood pumped by the right side and left side of the heart can differ in the presence of certain congenital heart malformations, these amounts are usually the same. CO is the product of heart rate (HR) and SV, the net amount of blood ejected by the heart in one cycle:
HR is an obvious determinant, as are the components of SV: preload, afterload, and contractility, which must all be considered when determining the cause of hypotension. CO can be measured clinically by thermodilution via a PA catheter and by transesophageal echocardiography (TEE). Less invasive devices to measure CO have been developed, including esophageal Doppler and pulse contour analysis (also see Chapter 20 ). Because the normal CO changes according to body size, cardiac index (CO divided by body surface area) is often used.
Tachycardia and bradycardia can cause hypotension if CO is decreased. The electrocardiogram (ECG), pulse oximetry, or physical examination can identify the presence of bradycardia or tachycardia. A determination of rhythm and an understanding of how rhythm affects filling pressures are essential for analyzing HR and its potential effects on hypotension. Loss of sinus rhythm and atrial contraction results in decreased ventricular filling. Atrial contraction constitutes a significant percentage of preload, even more so in patients with a poorly compliant ventricle. A slow HR may result in enhanced ventricular filling and an increased SV, but an excessively slow HR results in an inadequate CO in the setting of a stiff ventricle, making CO more HR dependent. Tachycardia may result in insufficient time for the LV to fill and result in low CO and hypotension.
Ejection fraction (EF) is the percentage of ventricular blood volume that is pumped by the heart in a single contraction (SV/end-diastolic volume [EDV]). Unlike SV, the EF does not differ based on body size, and an EF of 60% to 70% is considered normal. Hyperdynamic states such as sepsis and cirrhosis are reflected by an increased EF. Poor cardiac function is indicated by a reduced EF. Because CO can be maintained by increasing HR, the SV should be calculated to better assess cardiac function. However, with chronic dilated cardiomyopathy, the SV can improve despite the smaller EF.
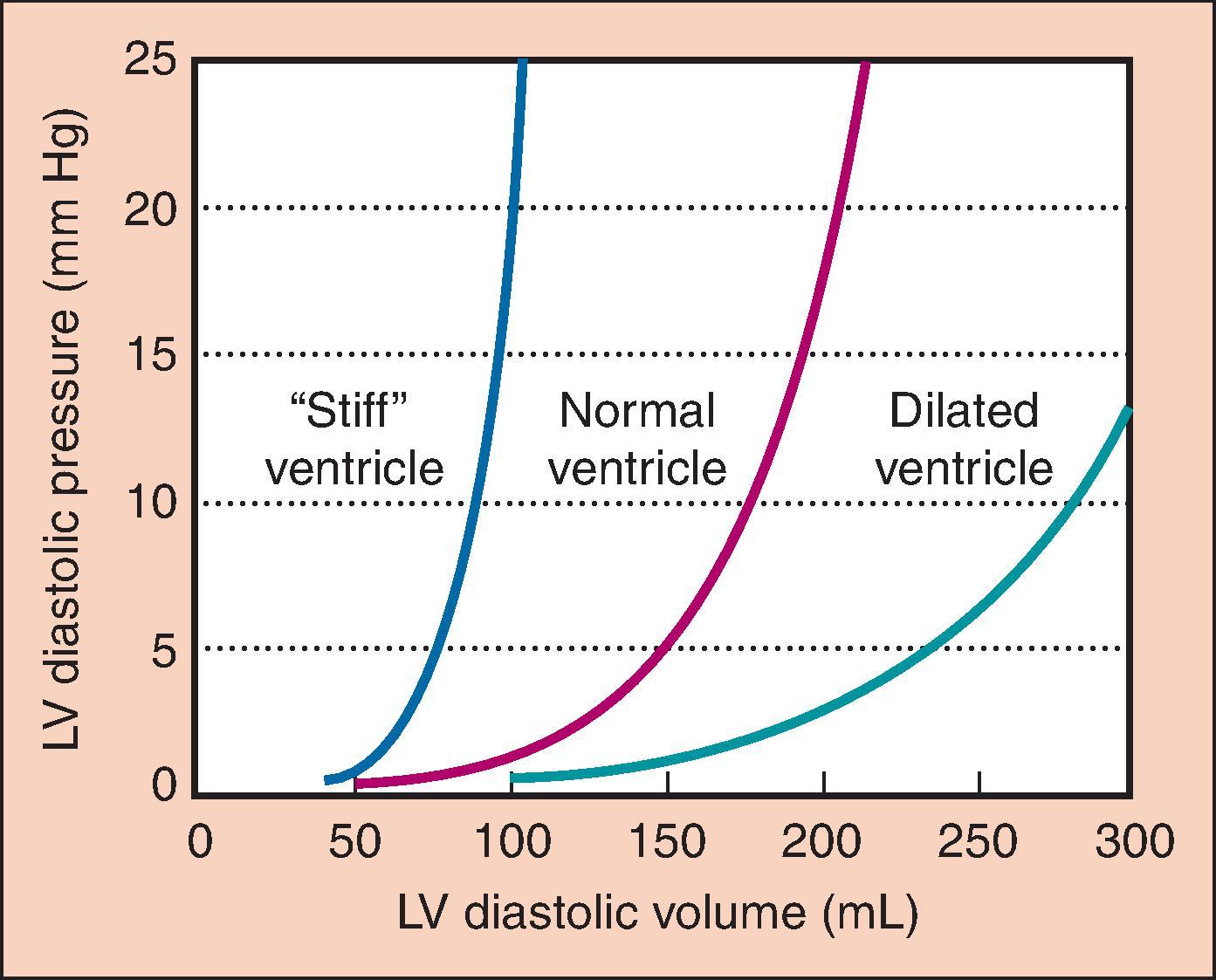
Preload refers to the amount the cardiac muscle is “stretched” before contraction. Preload is best defined clinically as the EDV of the heart, which can be measured directly with TEE. Filling pressures (e.g., left atrial [LA] pressure, pulmonary capillary wedge pressure [PCWP], pulmonary artery diastolic [PAD] pressure) can also assess preload. CVP measures filling pressures on the right side of the heart, which correlates with filling pressures on the left side of the heart in the absence of pulmonary disease and when cardiac function is normal. By using a balloon to stop flow in a PA, pressure equilibrates within the system so that PCWP is nearly equivalent to LA pressure and reflects the filling pressure of the left side of the heart. The relationship between pressure and volume of the heart in diastole is depicted by ventricular compliance curves ( Fig. 5.1 ). With a poorly compliant heart, normal filling pressures may not produce an adequate EDV. Likewise, trying to fill a “stiff” LV to a normal volume may increase intracardiac and pulmonary capillary pressures excessively.
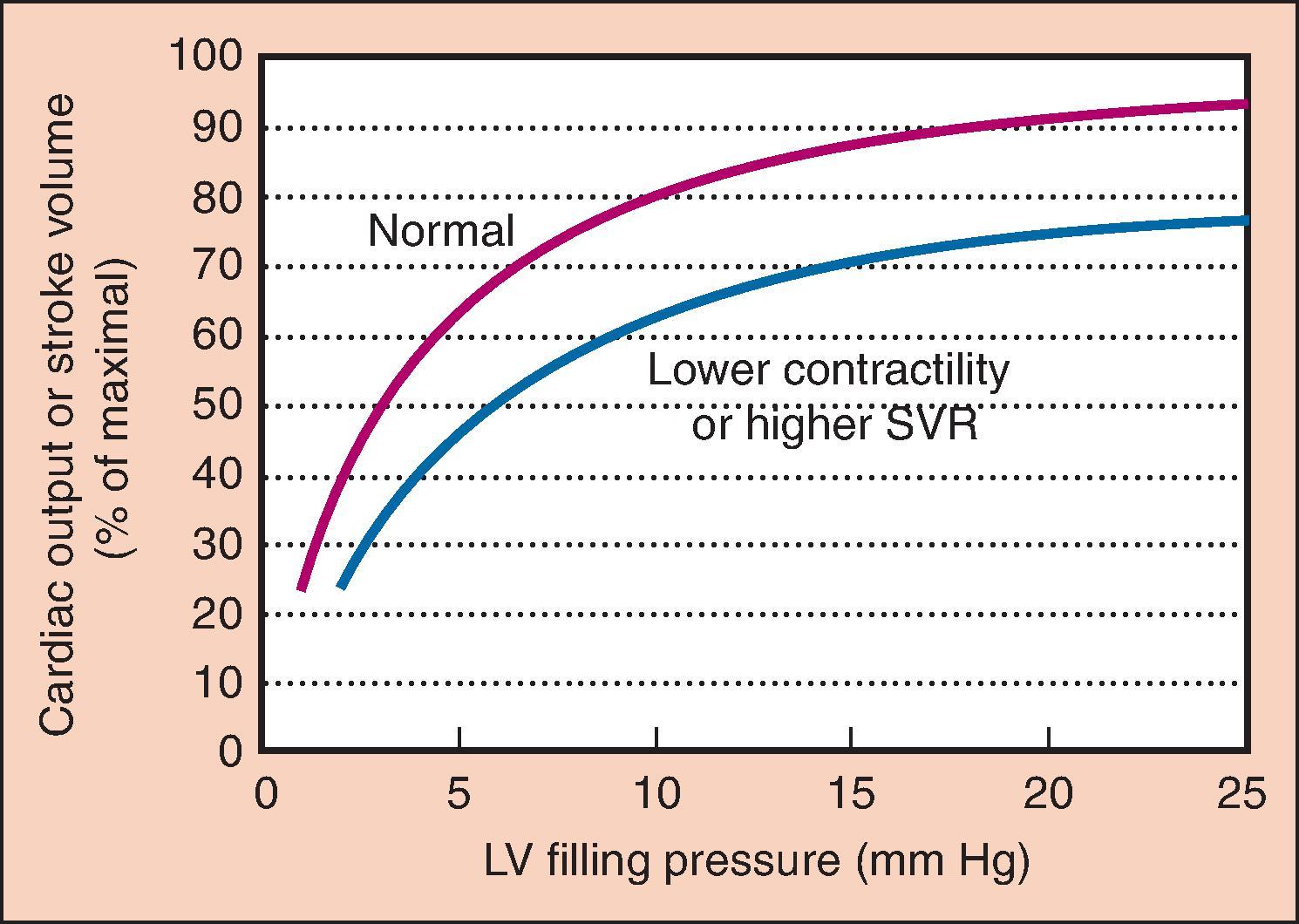
The Frank–Starling mechanism, a physiologic description of the increased pumping action of the heart with increased filling to an ideal volume, highlights the importance of preload. For a given ventricle, achievement of the ideal preload results in increased contraction necessary to eject added ventricular volume, resulting in a larger SV and similar EF. Reduced ventricular filling, as in hypovolemia, results in reduced SV. Small increases in preload may have dramatic effects (“volume responsiveness”) on SV and CO ( Fig. 5.2 ). At higher points on the curve, little additional benefit is derived from increases in preload; for patients with heart failure, the increase in preload can be detrimental.
Causes of low preload can be broken down into absolute (hypovolemia) and relative (venodilation and obstruction). Hypovolemia may result from hemorrhage or fluid losses. Venodilation occurs with general anesthesia and may be even more prominent in the presence of neuraxial anesthesia (also see Chapter 17 ). Tension pneumothorax and pericardial tamponade prevent ventricular filling because of increased pressure around the heart, obstructing blood flow and preventing the establishment of appropriate filling pressures. Thus cardiac tamponade may exist even in the setting of normal or increased CVP. Patients with low preload may manifest systolic pressure variation (SPV), which describes changes in SBP with tidal breathing or ventilation that can be observed on an arterial blood pressure tracing (also see Chapter 20 ). The extreme form of this is pulsus paradoxus, a marked decrease in SBP during the inspiratory phase of tidal breathing. Pulse pressure variation (PPV) [(PP peak − PP nadir )/PP average ] is analogous to SPV but requires computer calculation. Both SPV and PPV are also useful in identifying hypovolemia and are more sensitive and specific indicators of intravascular volume responsiveness than filling pressures such as CVP. However, when using low tidal volume ventilation, especially in patients with poor lung compliance (e.g., acute respiratory distress syndrome [ARDS]), SPV and PPV may not reliably predict fluid responsiveness unless tidal volume is temporarily increased (so-called tidal volume challenge ).
Additionally, pathologic problems on the right side of the heart may prevent filling of the LV. Right heart failure, pulmonary embolism, and other causes of pulmonary hypertension may prevent the right side of the heart from pumping a sufficient volume to fill the left side of the heart. With an overfilled RV (e.g., from hypervolemia and/or pump failure), the interventricular septum may be shifted, further constricting filling of the left side of the heart.
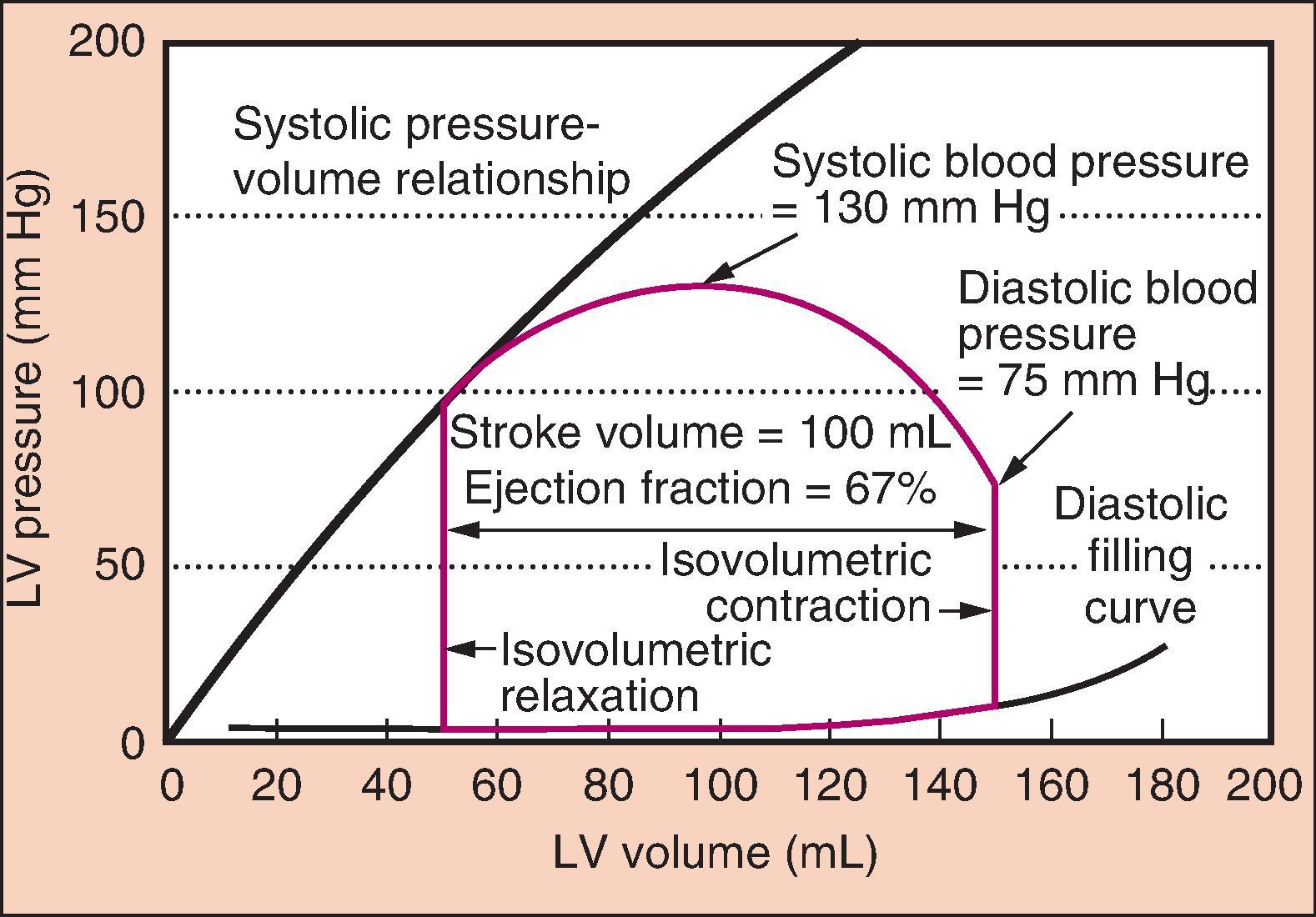
Contractility, or the inotropic state of the heart, is a measure of the force of contraction independent of loading conditions (preload or afterload). It can be measured for research purposes by the rate at which pressure develops in the cardiac ventricles (dP/dT) or by systolic pressure–volume relationships ( Fig. 5.3 ). Decreased myocardial contractility, alone or in conjunction with other causes discussed in this section, should be considered in the differential diagnosis of hypotension ( Box 5.1 ).
Myocardial ischemia
Anesthetic drugs
Cardiomyopathy
Previous myocardial infarction
Valvular heart disease (decreased stroke volume independent of preload)
Afterload is the resistance to ejection of blood from the LV with each contraction. For our purposes, afterload is largely determined by SVR and myocardial wall stress. When SVR is increased beyond normal values (900 to 1440 dyn/s/cm −5 ), the heart does not empty as completely, resulting in a lower SV, EF, and CO (see Fig. 5.2 ). High SVR also increases cardiac filling pressures, which may contribute to increased cardiac ischemia (described later) and increased wall stress. Wall stress is described using the law of Laplace: wall stress = P × r/T. In this equation P = ventricular transmural pressure, r = ventricular radius, and T = ventricular wall thickness. Low SVR improves SV and increases CO such that a low SVR is often associated with a higher CO ( Fig. 5.4 ).
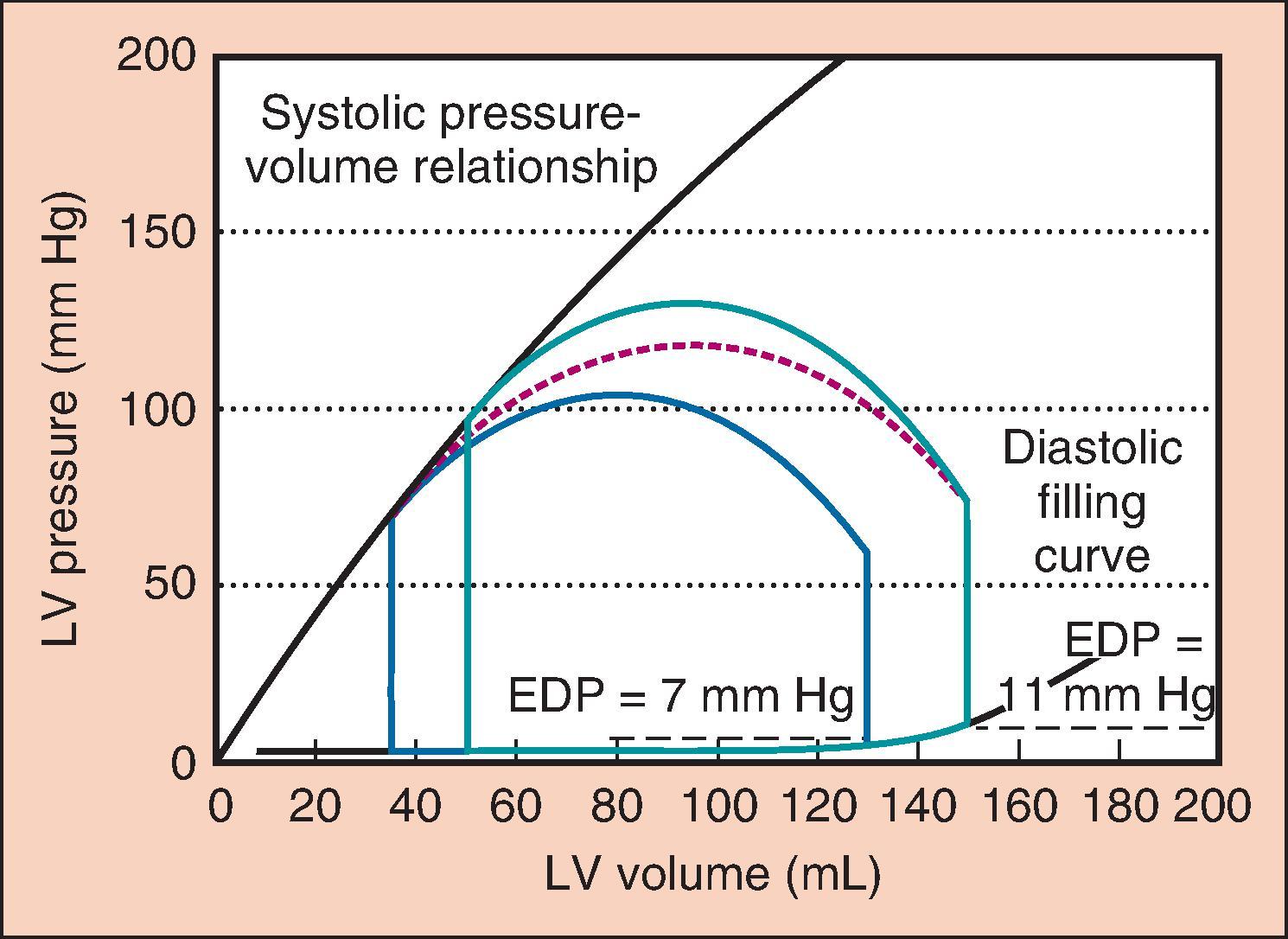
However, low SVR may also decrease cardiac filling pressures. This finding may suggest that preload rather than afterload is the cause of hypotension. Low SVR allows more extensive emptying and a lower end-systolic volume (ESV), one of the hallmarks of low SVR on TEE. With the same venous return, the heart does not fill to the same EDV, resulting in lower left ventricular filling pressures (see Fig. 5.4 ). A similar process occurs when the SVR is increased. Such stress-induced increases in cardiac filling pressures are more pronounced in patients with poor cardiac function. For any given patient, the goal is to find the SVR at which filling pressures allow the ventricle to operate on the most efficient portion of the Starling curve while not providing excessive afterload.
The cardiovascular regulatory system consists of peripheral and central receptor systems that can detect various physiologic states, a central “integratory” system in the brainstem, and neurohumoral output to the heart and vascular system. A clinical understanding of cardiac reflexes is based on the concept that the cardiovascular system in the brainstem integrates the signal and provides a response through the autonomic nervous system.
The heart and vascular systems are controlled by the autonomic nervous system. Sympathetic and parasympathetic efferents innervate the sinoatrial and atrioventricular nodes. Sympathetic nervous system stimulation increases HR through activation of β 1 -adrenergic receptors. Parasympathetic nervous system stimulation can profoundly slow HR through stimulation of muscarinic acetylcholine receptors in the sinoatrial and atrioventricular nodes, whereas parasympathetic nervous system suppression contributes to increased HR. Conduction through the atrioventricular node is increased and decreased by sympathetic and parasympathetic nervous system innervation, respectively. Sympathetic nervous system stimulation increases myocardial contractility. Parasympathetic nervous system stimulation may decrease myocardial contractility slightly, but it has its major effect through decreasing HR.
Baroreceptors in the carotid sinus and aortic arch are activated by increased systemic blood pressure that stimulates stretch receptors to send signals through the vagus and glossopharyngeal nerves to the central nervous system. The sensitivity of baroreceptors to systemic blood pressure changes varies and is significantly altered by long-standing essential hypertension. A typical response to acute hypertension is increased parasympathetic nervous system stimulation that decreases HR. Vagal stimulation and decreases in sympathetic nervous system activity also decrease myocardial contractility and cause reflex vasodilatation. This carotid sinus reflex can be used therapeutically to produce vagal stimulation that may be an effective treatment for supraventricular tachycardia.
The atria and ventricles are innervated by a variety of sympathetic and parasympathetic receptor systems. Atrial stretch (i.e., Bainbridge reflex) can increase HR, which may help match CO to venous return.
Stimulation of the chemoreceptors in the carotid sinus has respiratory and cardiovascular effects. Arterial hypoxemia results in sympathetic nervous system stimulation. The sympathetic response is a major factor in tolerance of mild to moderate hypoxemia. However, it is notable that more profound and prolonged arterial hypoxemia can result in bradycardia, possibly through central mechanisms. A variety of other reflexes causing bradycardia include increased ocular pressure (i.e., oculocardiac reflex), bradycardia with stretch of abdominal viscera, and the Cushing reflex (bradycardia in response to increased intracranial pressure).
Many anesthetics blunt cardiac reflexes in a dose-dependent fashion, with the result that sympathetic nervous system responses to hypotension are reduced. The blunting of such reflexes represents an additional mechanism by which anesthetic drugs contribute to hypotension.
The coronary circulation is unique in that a larger percentage of oxygen is extracted by the heart than in any other vascular bed, up to 60% to 70%, compared with the 25% extraction for the body as a whole. The consequence of this physiology is that the heart cannot increase oxygen extraction as a reserve mechanism. In cases of threatened oxygen supply vasodilatation to increase blood flow is the primary compensatory mechanism of the heart.
Coronary reserve is the ability of the coronary circulation to increase flow more than the baseline state. Endogenous regulators of coronary blood flow include adenosine, nitric oxide, and adrenergic stimulation. With coronary artery stenosis, compensatory vasodilatation downstream can maintain coronary blood flow until about 90% stenosis, when coronary reserve begins to become exhausted.
The perfusion pressure of a vascular bed is usually calculated as the difference between MAP and venous pressure, based on the Hagen–Poiseuille law:
where Q is flow, P 1 is upstream pressure, P 2 is downstream pressure, and R is resistance. Instantaneous flow through the coronary arteries varies throughout the cardiac cycle, peaking during systole. The heart is fundamentally different from other organs because the myocardial wall tension developed during systole can completely stop blood flow in the subendocardium. The LV is therefore perfused predominantly during diastole. The end-diastolic pressure in the left ventricle (LVEDP) may exceed CVP and represents the effective downstream pressure. Perfusion pressure to most of the LV is therefore DBP minus LVEDP. Thus elevated LVEDP, as is seen in decompensated heart failure or extreme volume overload, may be associated with coronary ischemic events. The RV, with its lower intramural pressure, is perfused during diastole and systole.
The pulmonary circulation includes the RV, PAs, pulmonary capillary bed, and pulmonary veins, ending in the left atrium. The bronchial circulation supplies nutrients to lung tissue and empties into the pulmonary veins and left atrium. The pulmonary circulation differs substantially from the systemic circulation in its regulation, normal pressures ( Table 5.1 ), and responses to drugs. Use of a PA catheter to measure pressures in the pulmonary circulation requires a fundamental understanding of their normal values and their meaning. Pulmonary hypertension has idiopathic causes and may accompany several common diseases (e.g., cirrhosis of the liver, sleep apnea). It is associated with significant anesthetic-related morbidity and mortality rates and, like many disease states, requires a sound understanding of cardiopulmonary physiology to mitigate complications (also see Chapter 26 ).
| Value | CVP (mm Hg) | PAS (mm Hg) | PAD (mm Hg) | PAM (mm Hg) | PCWP (mm Hg) |
|---|---|---|---|---|---|
| Normal | 2–8 | 15–30 | 4–12 | 9–16 | 4–12 |
| High | >12 | >30 | >12 | >25 | >12 |
| Pathologic | >18 | >40 | >20 | >35 | >20 |
Pulmonary artery pressure (PAP) is much lower than systemic pressure because of low pulmonary vascular resistance (PVR). Like the systemic circulation, the pulmonary circulation accepts the entire CO and must adapt its resistance to meet different conditions.
Determinants of PVR are different from SVR in the systemic circulation. During blood flow through the pulmonary circulation, resistance is thought to occur in the larger vessels, small arteries, and capillary bed. Vessels within the alveoli and the extraalveolar vessels respond differently to forces within the lung.
The most useful physiologic model for describing changes in the pulmonary circulation is the distention of capillaries and the recruitment of new capillaries. The distention and recruitment of capillaries explain the changes in PVR in a variety of circumstances. Increased PAP causes distention and recruitment of capillaries, increasing the cross-sectional area and decreasing PVR. Increased CO also decreases PVR through distention and recruitment. The reciprocal changes between CO and PVR maintain fairly constant pulmonary pressures over a wide range of CO values in nonpathologic states.
Just as cardiac contraction can affect coronary blood flow, alveolar distension can affect pulmonary blood flow. Lung volumes have different effects on intraalveolar and extraalveolar vessels. With large lung volumes, intraalveolar vessels can be compressed, whereas extraalveolar vessels have lower resistance. The opposite is true at very small lung volumes. Therefore higher PVR can occur at both large and small lung volumes. Increased PVR at small lung volumes helps to divert blood flow from collapsed alveoli, such as during one-lung ventilation.
Sympathetic nervous system stimulation can cause pulmonary vasoconstriction, but the effect is not large, in contrast to the systemic circulation, in which neurohumoral influence is the primary regulator of vascular tone. The pulmonary circulation has therefore been very difficult to treat with drugs. Previously, the mainstay of treatment included nitric oxide, an important regulator of vascular tone delivered by inhalation, in addition to prostaglandins and phosphodiesterase inhibitors (e.g., sildenafil), which are both pulmonary vasodilators. Although advances in pharmacologic treatments have been made and new receptors can be targeted (e.g., endothelin receptor antagonists, guanylate cyclase stimulators, and prostacyclins), effective pulmonary hypertension treatment remains challenging.
Hypoxic pulmonary vasoconstriction (HPV) is the pulmonary vascular response to a low alveolar oxygen partial pressure (P ao 2 ). In many patients HPV is an important adaptive response that improves gas exchange by diverting blood away from poorly ventilated areas, decreasing shunt fraction. Normal regions of the lung can easily accommodate the additional blood flow without increasing PAP. Global alveolar hypoxia, such as occurs with apnea or at high altitude, can cause significant HPV and increased PAP.
Anesthetic drugs such as the potent inhaled anesthetics can impair HPV, whereas commonly used intravenous drugs, such as propofol and opioids, demonstrate no inhibition of HPV. Calcium channel blockers may blunt HPV in the setting of preexisting V/Q mismatch. During surgical procedures requiring one-lung ventilation, HPV may play a role in the resolution of hypoxemia, although many other factors are also important, including acid–base status, CO, development of atelectasis, and concomitant drug administration (also see Chapter 27 ).
Pulmonary emboli obstruct blood vessels, increasing the overall resistance to blood through the pulmonary vascular system. Common forms of emboli are blood clots and air, but they also include amniotic fluid, carbon dioxide, and fat emboli.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here