Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The incidence of CKD is increasing in the United States primarily driven by the rising incidence of obesity-related disease, such as hypertension and diabetes, and shifts in age and racial demographics. The complications of CKD intensify as GFR declines, and therefore an understanding of the advantages and limitations of various methods of assessing and anticipating progression of kidney disease is important. Our discussion of the pathophysiology and treatment of patients with CKD is based on the concept that the cause of the morbidity and mortality occurring in CKD patients results from a reduced ability to excrete nitrogenous, non-nitrogen-containing metabolites and ions. It follows that restricting the precursor of treatment reduces signs and symptoms of CKD and is nutritionally safe as long as co-morbid conditions are considered. We describe how diet can influence mechanisms that contribute to the progression and complications of CKD, such as the accelerative effect of hypertension and proteinuria, abnormalities in protein metabolism and the potential role that uric acid accumulation plays in the progression of CKD. We discuss how uremic toxins in significant proportion derived from intestinal flora, contribute to dysregulation of physiologic processes and influence inflammation. Dysregulation of insulin signaling, acidosis and the development of inflammation contribute to accelerated muscle wasting, frailty and cardiovascular disease associated with CKD. Thus, it is vital to take advantage of strategies to prevent complications of CKD and delay the need for dialysis, especially since early dialysis has not been found to improve survival, clinical outcomes or quality of life.
Keywords
protein metabolism, dietary protein, protein restriction, low salt diet, CKD progression, uric acid, insulin resistance, muscle wasting, metabolic acidosis, uremic toxins
The concept of chronic kidney disease (CKD) continues to evolve from a common pathway initiated by unrelated diseases into a number of complications and adaptations that affect the function of virtually every organ. Key concepts are that these complications increase in severity as glomerular filtration rate (GFR) declines and their severity can be modified by modifying the diet and by treating each part of the syndrome of CKD. This concept of CKD allows for individual variations in the manifestations of the complications of CKD related to the primary kidney disease and/or an individual’s genetic and environmental background. The increasing number of patients with CKD and especially the growing number of elderly, diabetic, and minority populations, leads to morbidity and mortality concerns as well as the increasing costs of treating patients with end-stage renal disease (ESRD) when dialysis or transplantation are required to sustain life. Recent information underlines the necessity of employing established methods of treating CKD and preventing its complications. At least three groups of investigators have evaluated the hypothesis that “early dialysis” should be initiated in CKD patients. Their analyses demonstrated that early dialysis does not improve survival, clinical outcomes or quality of life.
The flood of new information about the pathophysiology of CKD (e.g., hypertension, the renin-angiotensin system, renal hypertrophy, electrolyte disturbances, nephron adaptations, and mineral metabolism, among others) has created separate fields of study. There also has been identification of mechanisms associated with the complications of CKD such as the contribution of the gastrointestinal tract to the generation of uremic toxins, the influence of erythropoietin on morbidity and mortality, the abnormalities in protein metabolism and the potential role that acid accumulation plays in the progression of CKD. Epidemiologic reports have emphasized the risks of CKD patients for the development of cardiovascular diseases and a link with the impending world wide increase in diabetes. These reports also emphasize that new strategies for slowing progression of CKD and mitigating its complications (e.g., cardiovascular disease, death) must be developed since a randomized, clinical trial has confirmed earlier reports indicating that initiating dialysis “early” does not improve mortality expectations of CKD patients. Our major focuses are the pathophysiology of progressive CKD and the role of the diet in counteracting CKD complications. The latter focus is relevant because successful implementation of dietary measures can blunt the severity of complications of CKD and recent findings show that this type of therapy is safe.
The “gold standard” for assessing the amount of residual kidney function and the rate of loss of kidney function is the GFR. The measurement of GFR is classically, the urinary clearance of inulin, a 5200 Da polysaccharide which is filtered by the glomerulus, is neither reabsorbed nor filtered and has no extrarenal clearance (i.e., no metabolism or elimination by other organs). Unfortunately, measuring inulin clearance is cumbersome and expensive: trained personnel are required to infuse inulin in order to achieve a prolonged, steady-state plasma concentration and for obtaining precisely timed urine collection. In addition, the measurement of inulin is difficult. To minimize errors due to retained urine in the bladder, patients are given a water load and sonography can be used to confirm completeness of bladder emptying. To minimize changes in renal perfusion, patients should remain supine or be seated, standing only to void. Despite these precautions, the coefficient of variation of inulin clearance ranges from 5–10 ml/min per 1.73 m 2 in subjects with nearly “normal” GFR values; the variability is higher in patients with CKD. Sources of this error include variation in the steady-state concentration of inulin; since the common practice is to collect urine after two to three hours which may be insufficient time for complete distribution of inulin and hence, produces a degree of variability in the plasma inulin concentration. This is the reason that the inulin clearance calculated from the rate of inulin infusion (mg/min) divided by the plasma inulin concentration consistently exceeds inulin clearance calculated from the urinary excretion. The alternative explanation is that there is an extrarenal clearance of inulin and there is no concurrence why the two means of calculation differ.
To reduce the analytic error due to difficulties in measuring inulin in blood and urine, radiolabeled compounds that are cleared predominantly by glomerular filtration have been used. Generally, these compounds (e.g., 125I-iothalamate) are gamma-emitters so the error from variable quenching of beta-emitting compounds (e.g., 14C-inulin) is minimized. Fortunately, there is concordance between the clearances of inulin and 125I-iothalamate (or other radiolabeled compounds) over a wide range of GFR. To avoid the error related to incomplete urine collections, GFR has also been calculated from the plasma disappearance of an injected compound (e.g., radiolabeled compounds or compounds like iothalamate which can be measured by spectrophotometry). This method is based on the assumption that the plasma and renal clearance will be insignificantly different because there is minimal extrarenal clearance of the compound. There are at least two other assumptions: (1) that the plasma clearance is constant when blood is sampled; and (2) the duration of blood sampling must be sufficiently prolonged to ensure that the terminal, steady-state period of monoexponential loss of plasma radioactivity with time is present. The latter requirement is especially important for patients with advanced CKD because hours may be required to reach a constant. In patients with severe CKD, there also may be a small extrarenal clearance (e.g., intestinal secretion) which would not be detected while there can be a delay in distribution of the GFR marker in patients with ascites or edema.
The radionuclide agents most commonly used to assess GFR is Tc-99m-DTPA and 125 I-iothalamate in the U.S. and 51 Cr-EDTA in Europe. Overall, the correlation of these radionuclide markers and urinary inulin clearance is high (r >0.9 in most published studies) as is the use of nonradioactive iodinated compounds, such as iohexol. In addition, some have advocated estimating the entire plasma disappearance curve from a single plasma sample obtained after only a few hours following injection of a GFR marker. Unfortunately, this technique is decidedly less accurate compared to the multiple sampling techniques, especially when there is renal insufficiency. Lastly, gamma cameras have been used to estimate GFR from the disappearance of labeled GFR markers but this technique has errors greater than plasma-based techniques (up to 20% error). An advantage of this technique is it can provide an estimate of GFR in individual kidneys.
A single value of serum creatinine (SCr) is an unreliable estimate of creatinine clearance (or GFR) because it is a function of creatinine production and its clearance by the kidney and extrarenal means. Creatinine production is emphasized because it is directly proportional to lean body mass and hence, a muscular individual will have a higher serum creatinine than one who has lost muscle mass, even though they have the same creatinine clearance (CCr). Three other caveats are important in evaluating GFR from the serum creatinine. First, the rate of creatinine production is not constant, despite the fact that lean body mass does not vary (at least over short periods). In part, this occurs because creatine in meat is converted to creatinine by cooking extensively. Secondly, the kidney not only filters but also secretes creatinine and hence consistently exceeds GFR by a variable amount. Third, creatinine is degraded, presumably by bacteria in the gastrointestinal tract. This extrarenal clearance is small and averages 0.04 liters/kg/day. Consequently, it is difficult to detect until renal clearance is depressed. Specifically, creatinine removal by extrarenal creatinine clearance (i.e., a method of clearing the blood that does not depend on excretion by the kidney) affects estimates of creatinine production when SCr rises to ~6 mg/dL. At this level, the amount of creatinine degraded is significant and should be considered in interpretation of estimates of SCr in CKD patients. The amount of creatinine degraded in mg/kg/day can be calculated as 0.042 l/kg/d x serum creatinine in mg/L.
The inaccuracies in estimating GFR from SCr can be compounded by practices in the Clinical Laboratory. If the SCr standards are 1, 4, and 10 mg/dl, the accuracy of measuring SCr in the normal range (typically less than 1.2 mg/dl) will be compounded. Variability among clinical laboratories in the calibration of serum creatinine assays introduces another error in GFR estimates especially at high SCr levels. Efforts are underway to institute national standardization of creatinine using values determined by isotope-dilution mass spectrometry (IDMS). Selected equations used to estimate the GFR from SCr yield results that are satisfactory as long as the patient is in the steady state and the SCr exceeds 2 mg/dl ( Table 90.1 ; Fig. 90.1 ). Although it can be clinically useful, the practice of estimating GFR from SCr often introduces a significant degree of inaccuracy.
| Name of Equation | Formula |
|---|---|
| Cockcroft-Gault CCr | Men: CCr=[(14-age)×weight (kg)]/SCr×72; Women: CCr=([(14-age)×weight (kg)]/SCr×72)×0.85 |
| MDRD Study Equation | GFR (mL/min/1.73 m 2 )=186×(S cr ) −1.154 ×(Age) −0.203 ×(0.742 if female)×(1.212 if African-American) |
| IDMS-Traceable MDRD Study Equation | GFR (mL/min/1.73 m 2 )=175×(S cr ) −1.154 ×(Age) −0.203 ×(0.742 if female)×(1.212 if African American) |
| CKD-Epidemiology group (CKD-EPI) | GFR (mL/min/1.73 m 2 )=141×min(Scr/k, 1) a ×max(Scr/k, 1) −1.209 ×0.993 Age ×1.018 [if female]×1.159 [if black] |
| Schwartz Equation | GFR (mL/min/1.73 m 2 )=k (Height in cm)/Serum creatinine; k=0.33 in preemie infants; 0.45 in term infants to 1 year of age; 0.55 in children to 13 years of age and adolescent females; 0.70 in adolescent males |
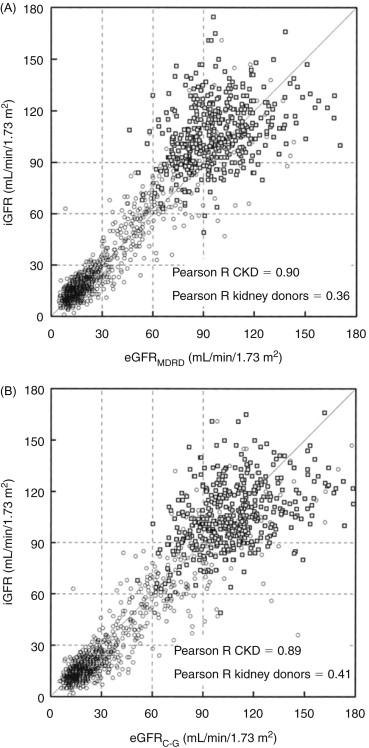
Since creatinine is secreted by proximal tubule cells, the 24-hour endogenous CCr usually exceeds inulin clearance but with advanced CKD, values of CCr and GFR become numerically closer even though the percentage difference between CCr and GFR increases. Using CCr as an estimate of GFR presents difficulties including the inconvenience of obtaining an accurate 24-hour urine collection. In addition, 24-hour CCr values have a high coefficient of variation: in one study of 119 healthy ambulatory subjects, the coefficient of variation was 26% and others reports that the variability varies from 6% to 22%. One reason for this variability is the day-to-day differences in creatinine excretion; among hospitalized patients, it varies by at least 10%. These problems have prompted some investigators to recommend abandoning the 24-hour CCr as an index of CKD severity. The same caveats apply to calculating the average of the 24-hour creatinine and urea clearances to estimate GFR, even though this average value corresponds closely to inulin clearance.
Another strategy is to measure CCr after giving cimetidine to inhibit creatinine secretion and hence, improve the estimate of GFR. Generally, 1.2 g of cimetidine completely suppresses creatinine secretion and lower doses yield incomplete blockade. Reportedly, with water loading and carefully timed urine collections, the cimetidine-adjusted CCr procedure yields values that have a coefficient of variation of approximately 8% in patients with GFR values ranging from 12 to 150 ml/min. Although this technique avoids using radioactive markers, it does require trained personnel to improve the accuracy of urine and blood collections.
Frustration with the variability of the 24-hour CCr has led to the widespread practice of estimating CCr from SCr. The equations rely on an estimated daily rate of creatinine production (and hence, excretion in the steady state). The widely used Cockcroft-Gault formula ( Table 90.1 ) is based on a value of creatinine production form the age, weight, and gender of the patient. However, it was derived from results obtained in “normal, hospitalized” patients, and hence, may not be appropriate for patients with CKD. It also does not include a value for extrarenal clearance (see above) although for SCr values below 6 mg/dL, this variable has a minimal impact on the estimates of creatinine production. Creatinine production in milligrams per kilograms per day has also been estimated as 28–0.2 A, and for women, as 23.8–0.17 A where A is the age of the subject in years. Besides using these equations to estimate CCr and hence, GFR, some investigators have calculated creatinine production as a means of estimating loss of muscle mass. Since there are no independent means of evaluating muscle mass, the reliability of this method is unknown. Regardless, creatinine production in mg/kg/day in men can be predicted as (28–0.2A)+(0.04×SCr×10) and for women, as (23.8–0.17×SCr×10). Using these formulas, the measured 24-hour CCr of uremic adults with stable serum creatinine values above 8 mg/dl was estimated to within 1.5 ml/min. It is important to recognize the assumptions underlying these estimates (and those accompanying the Cockcroft-Gault equation): they were derived from a homogenous cohort of predominantly Caucasian males without kidney disease and may not be appropriate for individuals of other races or groups. This shortcoming was uncovered when the MDRD formulas were studied in patients in China and Japan. Secondly, the Cockroft and Gault equation can systematically overestimate the GFR because of tubular secretion of creatinine.
The Modification of Diet in Renal Disease (MDRD) Study was a multicenter, randomized, controlled trial to evaluate the effectiveness of dietary protein restriction plus strict blood pressure control on the progression of renal insufficiency. The outcome was based on measurements of GFR estimated from urinary clearances of 125 I-iothalamate. Many other variables were collected (e.g., age, gender, weight, serum albumin, serum creatinine, etc.) and used to develop equations that predicted the measured 125 I-iothalamate clearance. The initial MDRD equation was found to yield a correlation of r 2 =91% ( Table 90.1 ; Eq. 90.1). In this equation, SUN is serum urea nitrogen and UUN is urine urea nitrogen. A revised MDRD equation ( Table 90.1 ; Eq. 90.2) is based on demographic assessments and serum variables only (yielding a maximal R2 of 90.3%). The most widely used modified MDRD equation was derived to avoid including serum values of albumin and urea nitrogen ( Table 90.1 ; Eq. 90.3). The latter equation has almost the same predictive ability and its use is fully supported by the National Kidney Disease Education Program (NKDEP) of the National Institutes of Health (NIH), the National Kidney Foundation (NKF), and the American Society of Nephrology (ASN). In fact, it is widely used as a means of estimating the severity of kidney disease based on a conclusion that patients with an estimated GFR below 60 ml/min/1.73 m 2 have a high risk of progressive CKD and should be treated to postpone or avoid ESRD.
There are caveats associated with using the MDRD equation: firstly, the equations were derived from results of measurements in only two ethnic groups, Caucasian and African Americans. Subsequent reports indicate that the equation does not accurately estimate GFR in Chinese, Japanese and Brazilian patients. In short, the ability of these equations to predict an individual’s GFR from SCr, etc. in other races/ethnicities will introduce errors. Furthermore, a recent analysis uncovered that measurements of the urinary clearance of 125 I-iothalamate (the reference GFR in the MDRD Study) had stable average values, but substantial variability across visits. Although it is known that GFR in individuals varies throughout the day and that repeated measures of GFR may better reflect an individual’s true GFR, there is inherent variability in GFR measured using iothalamate. This analysis points to another difficulty in interpreting GFR values and the severity of kidney disease based on SCr plus other factors present in an individual patient. An important factor is the age of the patient because those of >65–70 years comprised a small fraction of the MDRD study group and very few of ≥80 years were studied. It is critical to recognize that aged patients may have a depressed GFR even when SCr is within reference range. Regardless, the MDRD equation overestimates eGFR in the elderly. Besides the inaccuracies associated with different ethnic groups, there are serious deficiencies in the predictive value of these equations in people with obesity and normal kidney function.
In a study of adults from the Netherlands, Vervoort et al. compared the inulin clearance of 46 controls and 46 uncomplicated diabetic patients with predicted GFRs based on the MDRD and Cockcroft-Gault equations. The difference between predicted values and the measured GFR was significant (the Cockcroft-Gault formula had a 9.0 ml/min/1.73 m 2 difference while the MDRD equation had a 10.7 ml/min/1.73 m 2 difference from the measured inulin clearance). Secondly, Lin et al. found that the MDRD equation is decidedly less accurate in subjects without kidney disease when compared to GFR measured as 99mTc clearance; the MDRD equation systematically underestimated GFR while the Cockcroft-Gault formula overestimated the GFR. The Schwartz formula is commonly used to estimate GFR in children. Other equations used in estimating the GFR in CKD patients are listed in Table 90.1 .
In summary, the GFR can be predicted from formulas that are based on SCr and characteristics of a patient with CKD. The prediction equations exhibit errors and must be used with an understanding of the sources of variability, including the influence of obesity, race and age. Specifically, no single equation will reproducibly predict the GFR of an individual patient and this is especially true for subjects with minimal loss of kidney function or aged patients. Presumably, standardization of the measurement of creatinine and the use of a range of “standards” in a creatinine assay will be associated with improvements in the accuracy of the equations. But, it also is clear that GFR varies from hour to hour and is not constant day to day. These shortcomings do not invalidate the calculations because the estimation of GFR provides a means of identifying patients at risk for developing increasingly severe renal insufficiency and a method for detecting differences in rates of loss of kidney function (see below).
The estimated GFR based on the MDRD equation has increased the awareness of CKD in both physicians and patients. There is, however, concern about assigning a CKD diagnosis if there is no kidney disease. The concern has prompted The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) to derive another designed to improve the detection of CKD in adults with early/moderate kidney disease. This new equation ( Table 90.1 ) is based on a method of measuring creatinine that is traceable to standards established by isotope dilution mass spectroscopy (IDMS) and in patients with normal or mildly decreased GFR, it appears to yield a more accurate estimate of GFR compared to the MDRD Study equation. Importantly, when the CKD-EPI equation was used to analyze the NHANES database, it was concluded that the prevalence of CKD in U.S. adults was 11.5% compared to the estimate of 13.1% derived with the MDRD Study equation. Although this difference may seem small, the number of individuals predicted to have CKD would change from roughly 26 down to 23 million and thus may have significant public health implications.
Cystatin C is a low molecular-weight protein (13,000 Da) that is produced by all nucleated cells and acts to inhibit cysteine proteases. It has been used as an estimate of the severity of CKD because a high level of cystatin C is associated cardiovascular disease and may even be more closely associated than SCr. As a marker of GFR, however, it has shortcomings. A factor that estimates glomerular filtration should be produced as a constant rate, should not be removed by organs other than the kidney, and should be measured by a reliable method. Cystatin C fulfills some but not all of these requirements. Reportedly, the production rate of cystatin C is unaffected by age, gender, and muscle mass, while creatinine production is affected by all three. However, cystatin C production was not estimated using standard techniques but rather indirect estimates of production. Moreover, reports indicate that smoking, evidence of inflammation, and catabolic conditions affect cystatin C production. Second, although cystatin C is filtered through the glomerulus and is reabsorbed and catabolized by the proximal tubule, it is not known if other epithelial cells absorb and degrade cystatin C; it is evident that the extrarenal clearance of cystatin C is substantial. Third, cystatin C measurements employ an antibody-based technique, and there is controversy concerning which method is “most accurate.” Conclusions about the impact of CKD as assessed by serum cystatin C in patients is principally based on the conclusion that measured GFR has a higher correlation coefficient with serum cystatin C compared to GFR estimated from the reciprocal of serum creatinine. For example, in adults over 65 years of age living in four different areas in the US, it was found that in a comparison with serum creatinine, a high serum cystatin C level was correlated more closely with the number of deaths and cardiovascular events (stroke, myocardial infarction and congestive heart failure) occurring seven years later. Although it is known that CKD is associated with cardiovascular disease, the association between serum cystatin C and stroke, myocardial infarction and congestive heart failure, could be related to factors affecting cystatin C production and elimination that are independent of kidney function since there was no information about events and treatments occurring in the seven-year interval following the initial, baseline measurement. Thus, serum levels of cystatin C may be a harbinger of future adverse events but whether this can be attributed to CKD is unsettled. A related question is whether cystatin C accurately reflects the rate of loss of kidney function in patients with progressive renal insufficiency. Some have reported that serum cystatin C does reflect changes in GFR, but others conclude that the reciprocal of serum cystatin C is inferior or that there is no statistical difference between the analysis of changes in reciprocal serum creatinine and serum cystatin C. The association between serum cystatin C and GFR is more complex during pregnancy and in infants and children. It was proposed that serum cystatin C may have a higher sensitivity for detecting a low GFR compared to serum creatinine in patients with cirrhosis but in patients with chronic liver disease, neither serum cystatin C nor creatinine reliably predicts the GFR. The role of cystatin C continues to evolve but to date, it lacks evidence for being a precise measure of kidney function.
β-2-microglobulin is another endogenous protein that undergoes glomerular filtration plus reabsorption and metabolism by the proximal tubule. Like serum cystatin C, the serum β-2 microglobulin has been suggested to be more sensitive to an early decrease in GFR compared to serum creatinine. However, the influence of extrarenal clearance and constant production are unsettled and tubule degradation of the protein rises when urine is acidic, so patients should be given sodium bicarbonate to raise the urine pH above 7 if β-2 microglobulin is used to monitor kidney function. Even less fully investigated markers include beta-trace protein and tryptophan glycoconjugate and additional experiments are needed before they can be recommended as a method of monitoring kidney function.
In conclusion, the most accurate estimate of kidney function is the measurement of GFR using inulin, radionucleotide-labelled compounds, or iodinated compounds like iodothalamate. Results of studies from large groups of patients indicate that the MDRD equation can be used but it is important to remember its limitations. They include variations that are associated with different ages, races, etc. as well as those associated with the measurement of serum creatinine. Other strategies for estimating the degree of dysfunction of the kidney include the CCr that has been estimated from predictions of creatinine production and serum cystatin C. The latter has been used to evaluate the risks of adverse outcomes of kidney disease, including cardiovascular disease. But, there are problems with using serum cystatin C as an estimate of kidney function since its production depends on several factors and there is the potential for extrarenal clearance of cystatin C. To screen populations or even individuals for the presence of CKD, the MDRD equation has been most widely evaluated and provides a reasonable approximation. During long-term followup evaluations, it should be remembered that serum creatinine is the major factor that changes with renal insufficiency so determining the rate of loss of estimated GFR with time is subject to the same influences as evaluating changes in the reciprocal of serum creatinine with time (see below). In diagnosing CKD, a combination of techniques along with sound judgment about the influence of variables such as age, gender, diet, etc. should be employed since CKD is a progressive disease associated with increased mortality.
The epidemiology of ESRD is based largely on information from the United States Renal Data System (USRDS), a geographically comprehensive, population-based ESRD registry that collects information from all Medicare-eligible patients (~90% of all incident U.S. patients) at the start of dialysis, and at death or transplantation. Despite the robust information contained in the USRDS, it should be remembered that there are legitimate concerns about the accuracy of the primary cause of kidney disease, comorbid conditions, and the causes of death present in the yearly report. The concerns arise because diagnoses are left to the judgment of the treating nephrologist who often does not have a tissue diagnosis. For example, few ESRD patients who have hypertension listed as the cause of kidney failure have in fact, satisfied uniform diagnostic criteria for this diagnosis making it possible that other diseases led to ESRD. There are similar concerns about the accuracy of comorbid conditions and causes of death.
Undeniably, USRDS data indicate there has been substantial growth in the number of patients enrolled in the Medicare-supported ESRD program: there were roughly 10,000 beneficiaries in 1973 and over 86,000 a decade later but by 2009, the prevalence of ESRD in the US had grown to approximately 527,000. Moreover, in 1980, the incidence rate for ESRD compiled after adjustment for age, race/ethnicity and gender was 76 cases per million (18,000 patients). In 2002, the rate was 338 cases per million (102,000 patients) but by 2007, the rate was 361 cases per million (110,000 patients). The seemingly relentless increase in the incidence of ESRD has declined from 8% per year in 1980 to below 1.5% per year in the years after 2000. This encouraging change may represent improvements in preventive care or some unidentified factors.
In the 2009 USRDS report, the primary cause of ESRD was diabetes, afflicting 54% of new patients, yielding an incidence rate of 155 per million population. In contrast, the incidence of ESRD from hypertension or other kidney diseases has not increased. The diabetes-associated incidence rate is 36% higher than a decade ago and it parallels the rising prevalence of diabetes in the general population: increasing from 5% in the 1988–1994 era to 7.6% during 2003–2006. The increase in diabetes and CKD is especially prevalent in African and Native American populations. Besides diabetes, other ESRD-associated epidemiologically relevant factors include aging of the dialysis patients. From 1999–2009, the prevalent ESRD population of 20–40 years of age has risen 9.7%. Among those 40–60 year of age, the prevalence had risen 54% and 61% for those beyond 70 years. Besides age, the incidence of ESRD is clearly racial-dependent; in 2007, the incident rates in African Americans and Native Americans were 3.7 and 1.8 times greater, respectively, than the rate in whites while the rate in the Hispanic population is 1.5 times higher than that of non-Hispanics. Among African Americans, the rate of new ESRD cases in 2007 reached 998 per million population or 3.7 times greater than the rate of 273 among whites. Likewise, in 2007, Hispanic patients had an incidence rate of 508 per million population (1.5 times greater than in non-Hispanic patients). Thus, the annual increase in incidence ESRD may be leveling but these racial factors may still increase the number of ESRD patients because of demographic trends; similar factors present in developing countries may also be active and increase the number of ESRD patients.
Guidelines from the U.S. National Kidney Foundation (NKF) have led to a widely adopted framework for defining CKD. CKD is a nonspecific term that does not indicate the cause of kidney disease but it is useful for improving communications with investigators, practitioners and the public. CKD is defined as kidney injury and/or impaired kidney function lasting 3 or more months. Kidney injury is signified by the presence of microalbuminuria or proteinuria, abnormalities in the urinary sediment (RBC, RBC casts, WBC, WBC casts, tubular cells, cellular casts, granular casts, oval fat bodies, fatty casts, or free fat) and/or abnormal radiographic evidence and of course, evidence from kidney biopsies. CKD is categorized into five stages according to the level of the estimated GFR (see Table 90.2 ). All patients with CKD are increased cardiovascular risk, but those with an estimated GFR ≤60 ml/min/1.73 m 2 are also at higher risk for a progressive decline in kidney function and as GFR declines, pathophysiologic changes increase in complexity and management becomes more varied and increasingly specialized. The NKF guidelines attempted to tie the stages of CKD to specific clinical abnormalities and suggested treatment goals. In Stages 1 and 2, efforts are needed to adjust dietary factors, control blood pressure and glycemia. It is also suggested that inhibitors of the renin-angiotensin-aldosterone system (RAAS) should be used to conserve kidney function and reduce the risk of death and cardiovascular disease. In Stage 3 CKD, efforts are increased to detect and manage anemia and renal bone disease while the diet is controlled to prevent the accumulation of unexcreted waste products. These products include the myriad of compounds associated with the metabolism of a high protein diet (e.g., guanidines, middle molecules, etc.) and ions (sodium, acid, phosphates, etc.) but moderate to severe kidney disease, dietary adjustments are needed because the damaged kidney does not eliminate these metabolites. In Stage 4 CKD, attention to the same topics is needed and the patient should be introduced to options for treating ESRD; if hemodialysis is chosen, the patient should be evaluated for placement of a vascular access. At this stage, it is especially important to adjust medication doses for the loss of kidney function. In Stage 5 CKD, the pathophysiologic and metabolic functions become more difficult to manage and the clinician will need special training and skills to maximize therapy. The importance of managing the complications of advanced CKD is emphasized because the alternative option of initiating dialysis “early” (i.e., before uremic symptoms are present) has been tested repeatedly and found to be wanting in terms of improving mortality/morbidity.
| Stage | Description | GFR (ml/min) |
|---|---|---|
| 1 | Kidney damage (marked by proteinuria or abnormal urine sediment) | >=90 |
| 2 | Early kidney disease | 60–89 |
| 3 | Moderate kidney disease | 30–59 |
| 4 | Advanced kidney disease | 15–29 |
| 5 | Established renal failure | <15 or dialysis |
Unfortunately, many patients are unaware of the presence of CKD and the factors needed to treat CKD appropriately. In one study, patient awareness of CKD was only 22% of patients in Stage 3 and 44.5% of those with Stage 4 CKD (these dismal percentages were independent of race/ethnicity, gender, or age. ) Undoubtedly, the lack of awareness contributes to the development of complications of CKD in those with advanced CKD including anemia, bone disease, loss of muscle mass, abnormalities in serum electrolytes, etc. Similar shortcomings of managing CKD are present in primary care practices and Nephrology clinics. Even when it is recognized that a therapy target has not been met, clinicians can fail to change management, a behavior present in other chronic conditions and known as “clinical inertia”.
Population-based studies of epidemiologic factors and CKD are largely based on the National Health and Nutrition Survey (NHANES). This survey includes results from representative samples of the U.S. population that were collected by the National Center for Health Statistics of the Centers for Disease Control and Prevention. For example, the prevalence of Stages 1 to 4 CKD estimated from the NHANES data that was collected between1999 to 2004 was found to be 13.1% (approximately, 26.3 million people) and varies according to the stage of CKD. As expected from information about the epidemiology of patients with ESRD (see above), the prevalence of CKD is greater among diabetic subjects vs patients without diabetes (40.2% vs 15.4%). Data from the NHANES III survey of those in different stages of CKD also identify significant racial and gender discrepancies. Based on analyses of serum creatinine values, evidence from both diabetic and nondiabetic subjects reveal that a GFR below 60 ml/min/1.73 m 2 was present in 5% of non-Hispanic whites, 3.4% of non-Hispanic blacks, 1% of Mexican Americans, and 2.2% of other race/ethnic groups. Among black and white NHANES III participants with a comparable prevalence of CKD, it was estimated that the risk of developing ESRD for African Americans was fivefold greater compared to other populations even when results were controlled for differences in age, gender, and the presence of diabetes. Analyses of this database revealed that the prevalence of proteinuria in the U.S. population aged 20 years and older was present in those without impaired kidney function. In men of this age group, a random albumin/creatinine ratio of 17 to 250 mg/g (or 25 to 355 mg/g for women was) was present in 10.5% of the population; this was also present in 63.2% of the people on reexamination two months later. Overt proteinuria (an albumin/creatinine ratio >250 mg/g for men and 355 mg/g for women was found in 1.1% of the U.S. population, including 0.5% of individuals with an estimated GFR >90 ml/min/1.73 m 2 , 1.2% of whom had an estimated GFR between 60 and 89 ml/min/1.73 m 2 , and 7.2% of those with a GFR between 15 and 30 ml/min/1.73 m 2 . Evaluation of the NHANES III data indicated that increasing age affected the prevalence of proteinuria (urine albumin/creatinine ratio) and diabetes was the most frequently associated diagnosis while hypertension was the second most common abnormality. The staging of CKD also affected proteinuria since nonhypertensive, nondiabetic people with an estimated GFR between 30 and 60 ml/min/1.73 m 2 and aged 60 to 79 years had a prevalence of albuminuria of 20.6% compared to 14.1% among individuals with an estimated GFR >60 ml/min/1.73 m 2 .
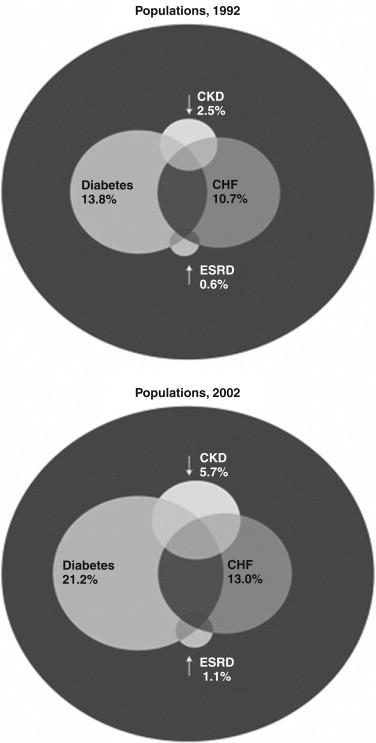
Patients with hypertension, diabetes mellitus, and cardiovascular disease, and family members of patients with ESRD are at high risk of developing CKD ( Fig. 90.2 ). Screening and health promotion can be cost-effective in such high-risk groups.
Hypertension. From the NHANES III results, the serum creatinine increased as the severity of hypertension rose. It is still not settled whether nonmalignant hypertension is a primary cause of ESRD or whether hypertension is caused by pre-existing kidney disease.
Diabetes. The USRDS has identified diabetes as the most common cause of ESRD, accounting for nearly 54% of all new cases of ESRD (USRDS ADR 2009). In a population-based study of Rochester, Minnesota, the risk of developing diabetic nephropathy among individuals with type 2 diabetes mellitus but no kidney disease, the incidence was 133 per 100,000 person-years. The cumulative incidence of diabetic nephropathy was similar for type 1 and type 2 diabetes mellitus: the 20-year cumulative risks were 27% for type 2 and 28% for type diabetes mellitus. Although there seems to be a declining incidence of diabetic nephropathy among individuals with type 1 diabetes mellitus (attributable to improved detection and care) but not for those with type 2 diabetes.
Cardiovascular Disease. CKD is associated with an increased risk of cardiovascular disease and adverse outcomes from it. Reports from more than 28 study populations reveal that cardiovascular disease patients had evidence of impaired kidney function; the average prevalence was 29.9%. The Valsartan in Acute Myocardial Infarction Trial (VALIANT) trial of participants evaluated from 0.5 to 12 days following an acute myocardial infarction complicated by heart failure and/or left ventricular dysfunction revealed that 11.3% had an estimated GFR<45 ml/min/1.73 m 2 and the overall prevalence of impaired kidney function was 33.5%. The baseline characteristics of the 1,120,295 participants in the Kaiser Permanente Renal Registry in Northern California aged 20 years and older revealed that the prevalence of an estimated GFR below 60 ml/min/1.73 m 2 was 6.4% of subjects with a history of diabetes mellitus, 7.5% of those with hypertension, 10.5% of those with coronary heart disease, and 12.5% of those with cerebrovascular disease. Other studies have essentially confirmed these associations.
Family History of ESRD. Individuals with a family history of ESRD have an increased prevalence of hypertension, diabetes, and CKD. Bergman et al. screened the first-degree relatives of patients with hypertensive ESRD and found that 65% of those in participating families had evidence of kidney disease, including those with a serum creatinine of ≥1.4 mg/dL. The same relationships occur in Canada.
Mortality. An analysis of nearly 28,000 health plan members with CKD defined as estimated GFR values below 90 ml/min per 1.73 m 2 on two occasions separated by at least 90 days revealed that during the ensuing five years, the cumulative risk of ESRD for CKD Stages 2, 3, and 4 were, respectively, 1.1%, 1.3%, and 19.9%, and the risk of death was 19.5%, 24.3%, and 45.7%. In another analysis of a large sample of patients, Go et al. found that the risk of all-cause mortality increased with decreasing GFR. In those with an estimated GFR of ≥60 ml/min per 1.73 m 2 there were 0.76 deaths per 100 person-years but 4.76 deaths per 100 person-years when the estimated GFR was 30–44 ml/min per 1.73 m 2 and 11.36 deaths per 100 person-years for individuals with a GFR 15–29 ml/min per 1.73 m 2 .
CVD Disease. Pre-existing cardiovascular disease in CKD patients increases the risk of morbidity and mortality. In a screening of 185 publications involving more than 550,000 subjects, 96% concluded there is a positive relationship between the presence of cardiovascular disease and CKD. Specifically, among individuals with an estimated GFR of 15–60 ml/min per 1.73 m 2 , the rate of myocardial infarction or fatal coronary heart disease was 13.9 per 1000 person-years vs 6.5 per 1000 person-years when the GFR was >60 ml/min per 1.73 m 2 .
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here