Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Pancreatic cancer is the fourth most common cause of cancer death in North America. The five-year survival rate is only 5.1% . The high lethality related to pancreatic cancer is due to a number of factors. It is biologically aggressive, it has profound effects on the host, and it is resistant to most cytotoxic agents. Moreover, early diagnosis is infrequent, and so resection (which represents the only chance for long-term control of this devastating disease) can be performed only in a minority of patients. Because of the limited success in diagnosing and treating pancreatic cancer, it is timely to consider alternative approaches to understanding the underlying pathophysiology. Pancreatic cancer is known to have metabolic effects on the host, and the tumor itself contains genetic aberrations with a number of metabolic consequences. Enhancing our understanding of the metabolic sequelae of pancreatic cancer therefore may lead to the development of new methods for diagnosis, as well as novel approaches to treatment.
With advances in technologies capable of the multiparametric interrogation of the metabolome, a more comprehensive view of the metabolic alterations that characterize pancreatic cancer is now possible. A number of groups have initiated such an effort. Some novel biological observations have been derived from metabolomics studies, although there is considerable potential to leverage on those initial observations. Metabolomic studies also have provided a foundation for development of novel diagnostic tests.
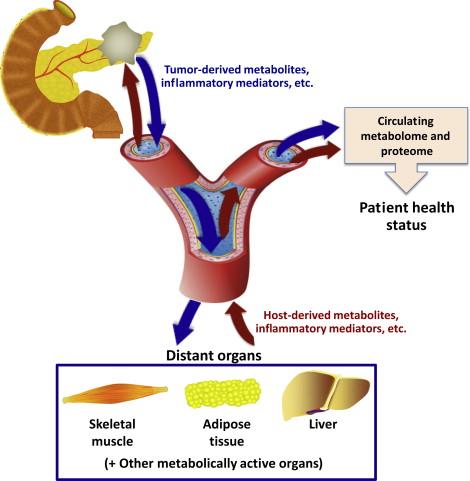
The metabolic derangements observed in any individual with pancreatic cancer are related to a multitude of factors. The diseased tissue (i.e., tumor cells and stroma) displays aberrant metabolism. Environmental and host factors that have predisposed the individual to pancreatic cancer in the first place also will be evident. Finally, established disease induces bioenergetic changes in adjacent and remote host tissues ( Figure 14.1 ).
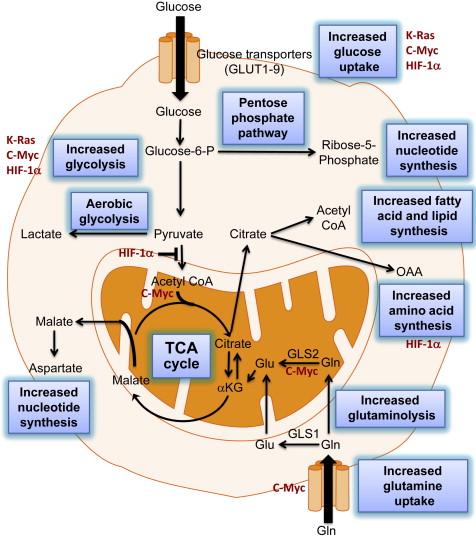
One of the hallmarks of a cancer cell is the reprogramming of energy metabolism . Large amounts of adenosine triphosphate (ATP) and substrate are required to support rapidly proliferating cells. Adaptations in metabolic pathways that typify cancer cells produce sufficient ATP as well as carbohydrates, proteins, lipids, and nucleotides to sustain the high metabolic demand. The classic example of metabolic reprogramming is the Warburg effect, described decades ago . In normal cells, in the presence of sufficient oxygen, glucose is processed through oxidative phosphorylation, generating a maximal amount of ATP. Glycolysis, a less efficient means to produce ATP, only becomes a primary means to metabolize glucose in hypoxic conditions. In contrast, in cancer cells, glycolysis is the dominant pathway for glucose metabolism regardless of oxygen supply. Although glycolysis is not as efficient as oxidative phosphorylation at generating ATP, it is a much more rapid means of ATP production, necessary to support multiple cellular divisions. This phenomenon of increased glucose processing in cancer cells forms the basis of using 18 F-fluorodeoxyglucose positron emission tomography (FDG-PET) to detect and monitor tumors . In addition to accelerated glycolysis, tumor cells have other characteristic features of metabolic reprogramming, each functioning to support the rapidly expanding biomass within the tumor. For example, glutamine uptake is enhanced, to replenish the tricarboxylic acid cycle; glutaminolysis also contributes to the production of acetyl coenzyme A for subsequent lipid biosynthesis, and increased fatty acid and lipid synthesis sustain synthesis of cell membranes and lipid derivatives.
The metabolic phenotype of cancer cells is regulated by both oncogenes and tumor suppressor genes (reviewed in ). In pancreatic cancer, mutations in the KRAS oncogene are found in more than 90% of pancreatic cancers . Tumors with KRAS mutations express high levels of glucose transporter-1 (GLUT1), providing the ability for enhanced glucose uptake and glycolysis, enabling survival in low glucose conditions . High levels of c-MYC expression are seen in almost 80% of pancreatic cancers . c-MYC overexpression accelerates glutaminolysis by several mechanisms, including increased expression of mitochondrial glutaminase (GLS) . Interestingly, c-MYC transcriptionally represses microRNAs mIR23a and mIR23b, resulting in increased expression of mitochondrial GLS . One additional effect of c-MYC overexpression is increased synthesis of acetyl-CoA in mitochondria, which subsequently increases histone acetylation and fatty acid biosynthesis . Hypoxia-inducible factor (HIF)-1α protein expression is seen in about half of pancreatic cancers as a result of intratumoral hypoxia and paracrine insulin ; hypoxia stabilizes the transcription factor and the protein . HIF-1 transcription factor activates numerous target genes (reviewed in ). HIF-1 transcription factor not only is a pivotal regulator of oxygen homeostasis but also encourages glycolysis, contributes to the metabolism of nucleotides and iron, and exerts additional effects on cellular bioenergetics through its mitogenic effects. Both c-MYC and HIF-1α increase the rate of transcription of some of the GLUT transporters (increasing glucose uptake by the cell) and hexokinase-2 . Thus, genetic alterations in the tumor can contribute to the metabolic phenotype of tumor cells and subsequently disturb host energy homeostasis ( Figure 14.2 ).
The metabolic phenotype of tumor cells can be influenced further by events at the transcriptional and protein levels. One example of this is the enzyme pyruvate kinase (PK), the enzyme that catalyzes the last step of glycolysis. In cancer cells, the M2-PK isoform (normally found in embryonic tissue) is the predominant PK isoform. Selective binding of M2-PK to tyrosine-phosphorylated peptides causes inhibition of M2-PK enzymatic activity , causing a shift in cellular metabolism to aerobic metabolism (the Warburg effect) . Impaired PK activity also leads to an accumulation of metabolites, preceding PK in the glycolytic pathway, which act as precursors for nucleic acids, amino acids, and phospholipids. This metabolic switch has been shown to provide a selective growth advantage to tumor cells .
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here