Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Channelopathies are disorders caused by ion channel dysfunction. Because of the great diversity of ion channel proteins and their expression in different tissues, channelopathies comprise a wide variety of clinical diseases ( Table 98.1 ), the discovery of which helps elucidate how ion channels function in both illness and health. The periodic paralyses —the first group of ion channel disorders characterized at a molecular level—defined the field of channelopathies, which now encompasses diseases not only in muscle but also in the kidney (Bartter syndrome), epithelium (cystic fibrosis), and heart (long QT syndrome), as well as neurons. Because muscles and neurons are electrical organs, it is not surprising that most channelopathies are associated with neurological disease. Despite significant heterogeneity, a pervasive feature of neurological channelopathies is a paroxysmal phenotype of various neurological presentations, encompassing myopathy, peripheral neuropathy, epilepsy, migraine headache, and episodic movement disorders. After a brief introduction to ion channels, this chapter describes disorders caused by congenital and acquired dysfunction of ion channels expressed in skeletal muscle, neurons and neuromuscular junction.
| Disease | Ion Channel | Gene | Chromosome |
|---|---|---|---|
| Muscular Channelopathies | |||
| Andersen-Tawil syndrome | Kir2.1 ∗ ; potassium (inward rectifier) | KCNJ2 | 17q23.1–q24.2 |
| Kir3.4; potassium (inward rectifier) | KCNJ5 | 11q24 | |
| Central core disease | Calcium (ryanodine receptor) | RYR1 | 19p13.1 |
| Congenital myasthenic syndromes | nAChR α 1 -subunit | CHRNA1 | 2q24–q32 |
| nAChR β 1 -subunit | CHRNB1 | 17p12–p11 | |
| nAChR δ-subunit | CHRND | 2q33–q34 | |
| nAChR ε-subunit | CHRNE | 17p13–p12 | |
| (nAChR anchoring protein: rapsyn) | RAPSN | 11p11.2–p11.1 | |
| Hyperkalemic periodic paralysis | Nav1.4; sodium α 4 -subunit | SCN4A | 17q23.1–q25.3 |
| Hypokalemic periodic paralysis | Cav1.1; calcium (L-type) | CACNA1S | 1q32 |
| Nav1.4; sodium α 4 -subunit | SCN4A | 17q23.1–q25.3 | |
| Malignant hyperthermia | Calcium (ryanodine receptor) | RYR1 | 19q13.1 |
| Cav1.1; calcium (L-type) | CACNA1S | 1q32 | |
| Myotonia congenita | Chloride | CLCN1 | 7q35 |
| Paramyotonia congenita | Nav1.4; sodium α 4 -subunit | SCN4A | 17q23.1–q25.3 |
| Potassium-aggravated myotonia | Nav1.4; sodium α 4 -subunit | SCN4A | 17q23.1–q25.3 |
| Neuronal Channelopathies | |||
| ADNFLE | nAChR α 4 -subunit | CHRNA4 | 20q13.2–q13.3 |
| nAChR β 2 -subunit | CHRNB2 | 1q21 | |
| KCa4.1; potassium (sodium-activated) | KCNT1 | 9q34.3 | |
| ADPEAF | (Potassium channel regulator) | LGI1 | 10q24 |
| Alternating hemiplegia of childhood | (Na + /K + -ATPase) | ATP1A2 | 1q21–q23 |
| BFNS | Kv7.2; potassium (M channel) | KCNQ2 | 20q13.3 |
| Kv7.3; potassium (M channel) | KCNQ3 | 8q24 | |
| BFNIS, BFIS | Nav1.2; sodium α 2 -subunit | SCN2A | 2q23–q24.3 |
| (Na + /K + -ATPase † ) | ATP1A2 | 1q21–q23 | |
| Childhood absence epilepsy | GABA A receptor γ 2 -subunit | GABRG2 | 5q31.1–q33.1 |
| GABA A receptor β 3 -subunit | GABRB3 | 15q11.2–q12 | |
| Cav3.2; calcium (T-type) | CACNA1H ‡ | 16p13.3 | |
| Cav2.1; calcium (P/Q-type) | CACNA1A ‡ | 19p13 | |
| Congenital stationary night blindness | Cav1.4; calcium (L-type) | CACNA1F | Xp11.23 |
| Deafness (nonsyndromic type 2) | Kv7.4; potassium | KCNQ4 | 1p34 |
| Episodic ataxia 1 | Kv1.1; potassium | KCNA1 | 12p13 |
| Episodic ataxia 2 | Cav1.4; calcium (P/Q-type) | CACNA1A | 19p13 |
| Episodic ataxia 5 | Calcium β 4 -subunit | CACNB4 | 2q22–q23 |
| Episodic ataxia 6 | (EAAT1 † ) | SLC1A3 | 5p13 |
| Familial hemiplegic migraine 1 | Cav1.4; calcium (P/Q-type) | CACNA1A | 19p13 |
| Familial hemiplegic migraine 2 | (Na + /K + -ATPase) | ATP1A2 | 1q21–q23 |
| Familial hemiplegic migraine 3 | Nav1.1; sodium α 1 -subunit | SCN1A | 2q24 |
| Familial temporal lobe epilepsy / febrile seizures | (Carboxypeptidase † ) | CPA6 | 8q13.2 |
| GEFS+ | Nav1.1; sodium α 1 -subunit | SCN1A | 2q24 |
| Sodium β 1 -subunit | SCN1B | 19q13.1 | |
| Nav1.2; sodium α 2 -subunit | SCN2A | 2q23–q24.3 | |
| Nav1.7; sodium α 9 -subunit | SCN9A | 2q24 | |
| GABA A receptor γ 2 -subunit | GABRG2 | 5q31.1–q33.1 | |
| GABA A receptor δ-subunit | GABRD | 1p36.3 | |
| Hereditary hyperekplexia | Glycine receptor α 1 -subunit | GLRA1 | 5q32 |
| Glycine receptor β-subunit | GLRB | 4q31.3 | |
| (Glycine transporter † ) | GLYT2 (SLC6A5) | 11p15.2–p15.1 | |
| JME | GABA A receptor α 1 -subunit | GABRA1 | 5q34–q35 |
| Calcium β 4 -subunit | CACNB4 ‡ | 2q22–q23 | |
| (R-type calcium channel regulator † ) | EFHC1 ‡ | 6p12–p11 | |
| Mental retardation, autosomal dominant | Glutamate receptor NR2B subunit | GRIN2B | 12p13.1 |
| PED | (GLUT1 † ) | SLC2A1 | 1p34.2 |
| PKD | (Proline-rich transmembrane protein 2 † ) | PRRT2 | 16p11.2 |
| PNKD with epilepsy | KCa1.1; potassium (BK) | KCNMA1 | 10q22.3 |
| PNKD without epilepsy | (PNKD protein † ) | PNKD | 2q35 |
| Primary erythermalgia | Nav1.7; sodium α 9 -subunit | SCN9A | 2q24 |
| Scapuloperoneal spinal muscular atrophy/congenital distal spinal muscular atrophy/CMT2C/HMSN2 | TRPV4 | TRPV4 | 12q24.1 |
| Spinocerebellar ataxia type 6 | Cav2.1; calcium (P/Q-type) | CACNA1A | 19p13 |
| Nonneurological Channelopathies | |||
| Bartter syndrome antenatal 1 | Na-K-2Cl cotransporter | SLC12A1 | 15q15–q21.1 |
| Bartter syndrome antenatal 2 | Kir1.1; potassium (inward rectifier) | KCNJ1 | 11q24 |
| Bartter syndrome 3 | Chloride | CLCNKB | 1p36 |
| Cystic fibrosis | Chloride | CFTR | 7q31.2 |
| Dent disease | Chloride | CLCN5 | Xp11.22 |
| FPHHI | Potassium (accessory subunit) | ABCC8 | 11p15.1 |
| Kir6.2; potassium (inward rectifier) | KCNJ11 | 11p15.1 | |
| Liddle syndrome 1 | Sodium (non-voltage-gated) | SCNN1A | 12p13 |
| Sodium (non-voltage-gated) | SCNN1B | 16p13–p12 | |
| Sodium (non-voltage-gated) | SCNN1G | 16p13–p12 | |
| LQT1 | Kv7.1; potassium | KCNQ1 | 11p15.5 |
| LQT2 | Kv11.1; potassium | KCNH2 | 7q35–36 |
| LQT3 | Nav1.5; sodium α 5 -subunit | SCN5A | 3p21–24 |
| LQT4 | (anchoring protein ankyrin-B † ) | ANK2 | 4q25–q27 |
| LQT5 | Potassium (accessory subunit) | KCNE1 | 21q22.1–q22.2 |
| LQT6 | Potassium (accessory subunit) | KCNE2 | 21q22.1 |
∗ Where appropriate, ion channel names are provided according to the International Union of Basic and Clinical Pharmacology Committee on Receptor Nomenclature and Drug Classification (NC-IUPHAR).
† These associated genes/proteins may not be ion channels; they may instead contribute indirectly to ion channel function, regulate neurotransmitter kinetics, or possess other or unknown functions.
One needs a basic understanding of channel structure and function before addressing channelopathies and their clinical manifestations. Ion channels are transmembrane glycoprotein pores that underlie cell excitability by regulating ion flow into and out of cells across the lipid bilayers of the cell membrane. They are composed of distinct protein subunits, each encoded by a separate gene. The categorization of most channels, depending on their means of activation, is as voltage-gated or ligand-gated . Changes in membrane potentials activate and inactivate voltage-gated ion channels. They are named according to the physiological ion preferentially conducted (e.g., Na + , K + , Ca 2+ , Cl − ). Ligand-gated ion channels respond instead to specific chemical neurotransmitters (e.g., acetylcholine, glutamate, γ-aminobutyric acid [GABA], glycine).
Distributed ubiquitously in excitable tissues, voltage-gated ion channels are critical for establishing a resting membrane potential and generating action potentials, especially in tissues where rapid conduction of messages is required (e.g., nerves, cardiac cells, or skeletal muscles). Most channels have a similar basic structure, consisting of one or more pore-forming subunits (generally referred to as α-subunits ) and a variable number of accessory subunits (often denoted β, γ, etc.). An α-subunit is composed of four homologous domains (I–IV) and typically has six transmembrane segments (S1–S6). The S4 segment has positively charged residues and serves as “a sensor.” The S5–S6 segments usually form the ion pore. These segments determine ion selectivity for the α-subunits, and voltage sensing is conferred by the S4 segment, while the remaining accessory subunits act as modulators.
Voltage-gated channels are “gated” with high sensitivity to changes in transmembrane potential. The conductance is tightly regulated by changes in conformations of the channel, as channels exist in one of three states: open, closed, or inactivated. Voltage-gated channels open (or activate) with threshold changes in membrane potential, then transition after a characteristic interval to either a closed or an inactivated state. From the closed state, a channel can reopen with an appropriate change in membrane potential. In the inactivated state, a change in membrane potential normally sufficient to open the channel is ineffective and the channels will not conduct current. Inactivation is both time and voltage dependent, and many channels display both fast and slow components of inactivation. Inactivation is a means of negative regulation of the channel, influencing electrical stability in excitable cells.
A neuron typically has a resting potential of –75 mV, with the intracellular side being negative relative to the extracellular space. When the resting potential reaches a threshold or more positive membrane potential of –55 mV, depolarization occurs, which leads to the production of an action potential. This is achieved by opening a specific ion channel—a voltage-gated sodium channel—in which case sodium ions will rush into the cells down the concentration gradient. In order to reach the action potential, the sodium channel changes its confirmation from a closed or resting state to an open state. When the membrane potential reaches its peak, about 40 mV, the sodium channels close and become inactive. Then, repolarization occurs, due to the opening of another voltage-gated channel, such as a potassium channel. This leads to a rapid return to the resting potential via an outflux of potassium ions (down the potassium concentration gradient). Before achieving a stable resting potential, the membrane potential becomes “hyperpolarized” for a short time. Slowly, the sodium channels return to the closed state from the inactivated state. While in the inactivated state, sodium channels are not responsive to voltage changes. However, in the closed state, they become sensitive to voltage changes again.
Different tissues and cells express different ion channels; thus, dysfunction of a specific ion channel can lead to a broad spectrum of phenotypes, depending on the tissue/cell type/ion channel involved. This is particularly important in the nervous system, where there is tremendous heterogeneity of the cells with regard to ion channel expression. Each subtype is encoded by a different gene, and its expression is highly cell specific.
Depending on the location within the channel, mutations could alter voltage-dependent activation, ion selectivity, or time and voltage dependence of inactivation. Thus, two different mutations within the same gene can result in dramatically different physiological effects. For example, a mutation that prevents or slows inactivation could lead to a persistent ionic current. Conversely, a mutation elsewhere in the same gene that prevents activation will decrease ionic current. Phenotypic heterogeneity describes how different mutations in a single gene cause distinct phenotypes. For instance, mutations in the skeletal muscle voltage-dependent sodium channel can result in hyperkalemic periodic paralysis, hypokalemic periodic paralysis, potassium-aggravated myotonia (PAM), or paramyotonia congenita (PMC; see Table 98.1 and Fig. 98.1 ). In contrast, genetic heterogeneity occurs when a consistent clinical syndrome results from a variety of underlying mutations in distinct genes. For example, familial hypokalemic periodic paralysis can result from distinct mutations in the SCN4A or CACNA1S genes.
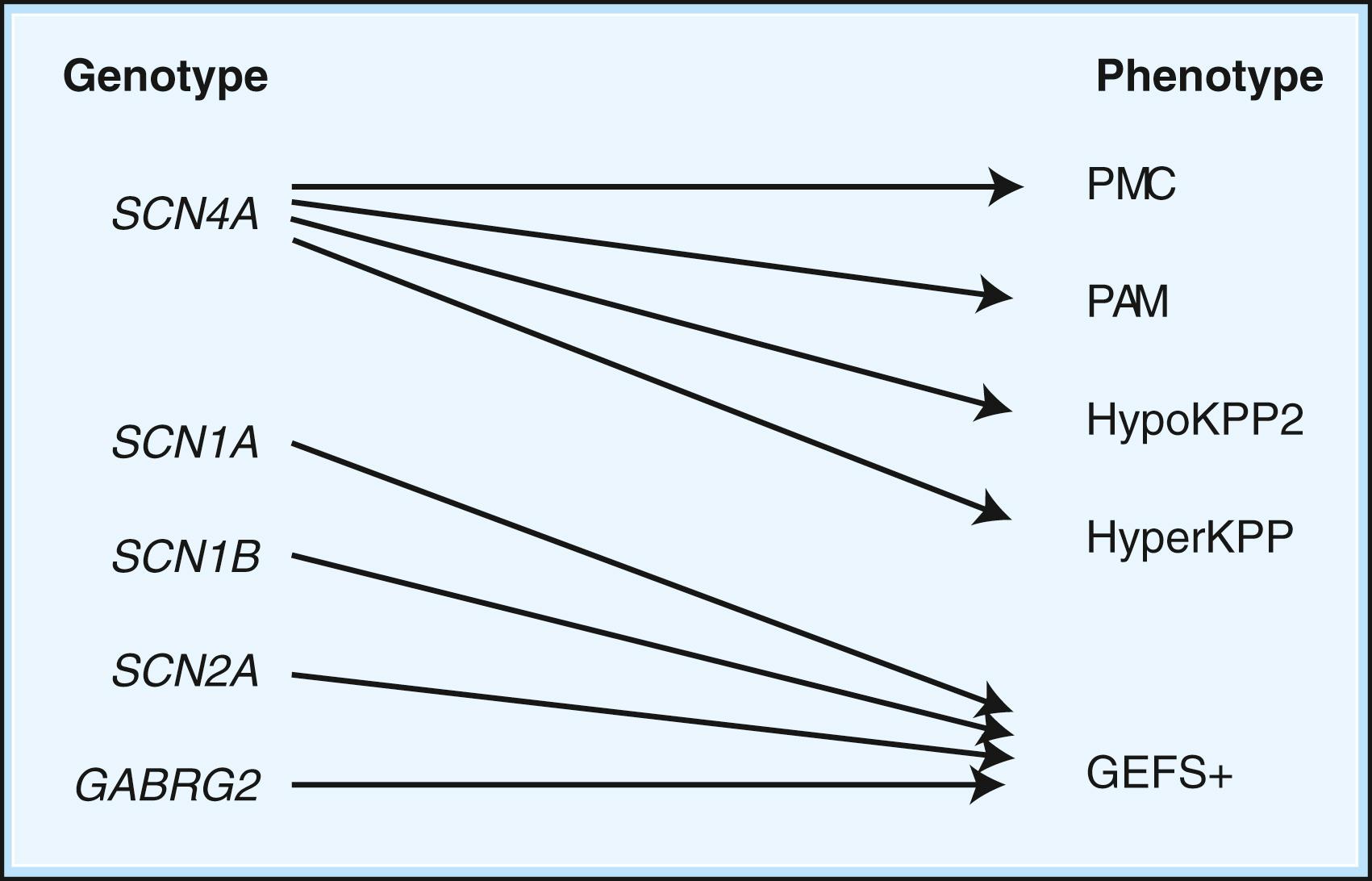
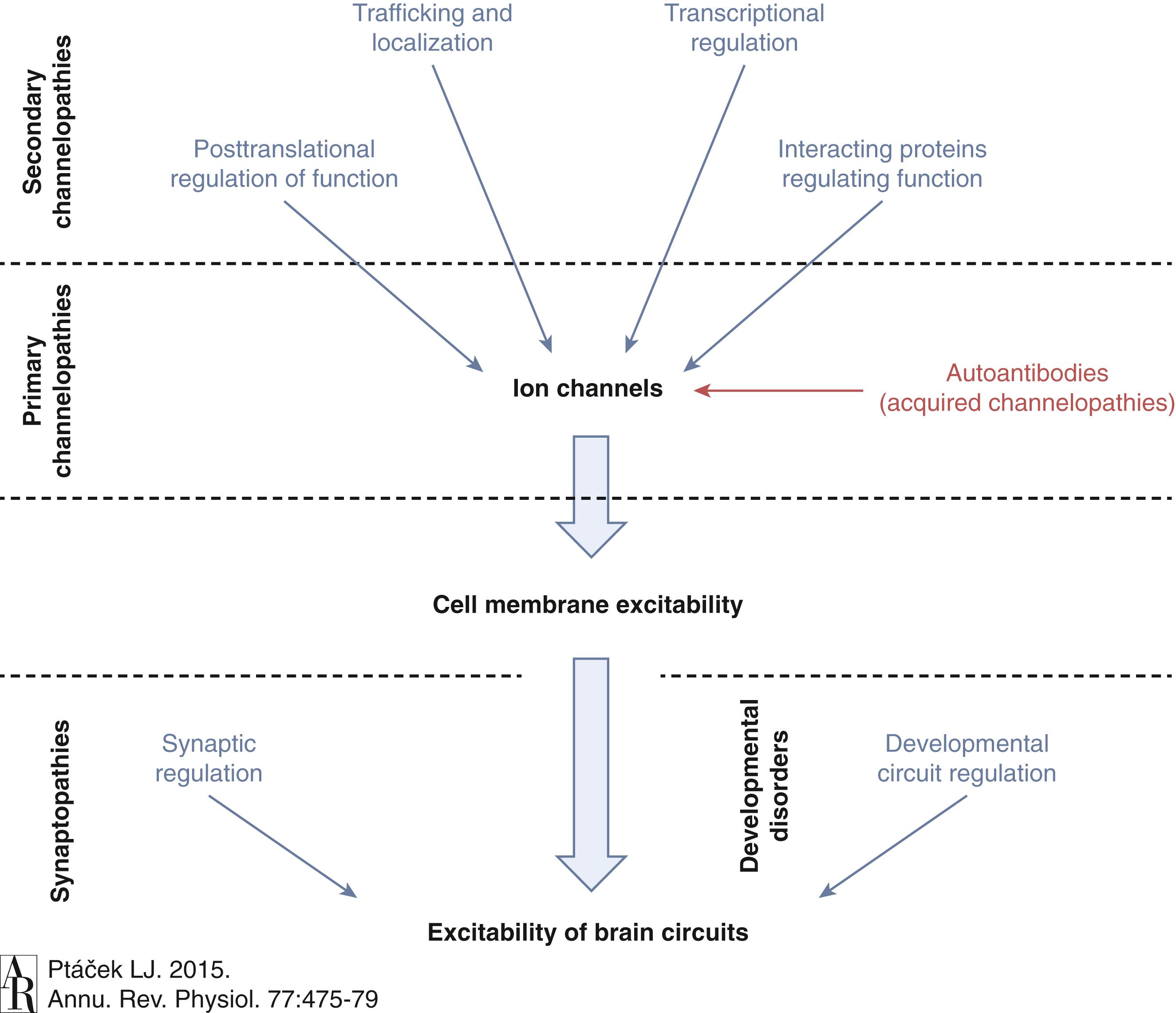
Ion channel mutations may lead to either “loss of function” or “gain of function” in each case. Generally speaking, loss-of-function mutations cause reduced permeability, whereas gain of function implies the gain of an abnormal function (e.g., increased permeability or altered selectivity vs. normal permeability in the wrong part of the cell). Furthermore, genetic channelopathies are not restricted to mutation of the channels, but other mutations involving regulatory, modifiers, posttranscriptional, and posttranslation changes can also result in ion channel dysfunction ( Fig. 98.2 )
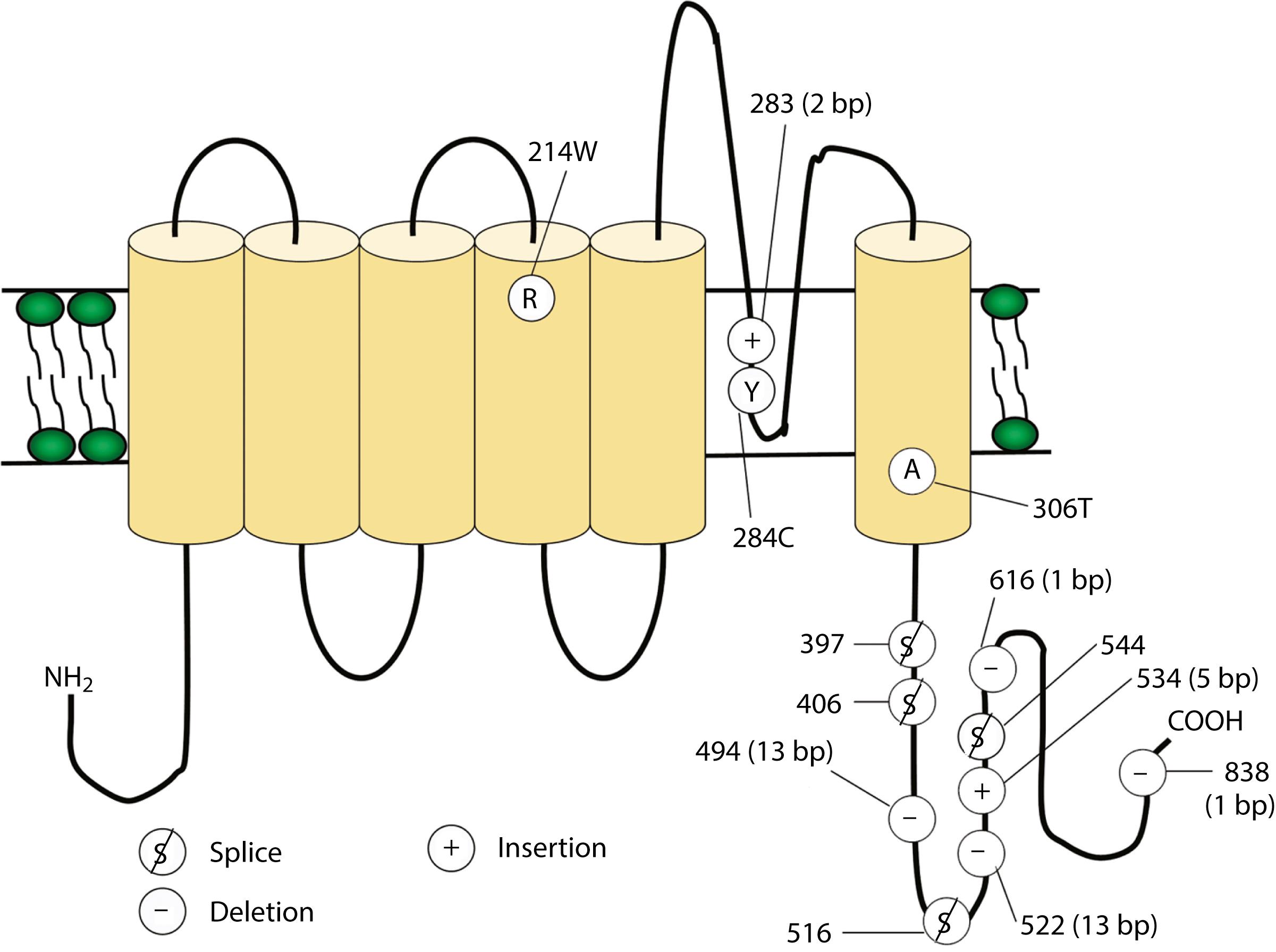
Voltage-gated potassium channels (VGKCs) consist of four homologous α-subunits that combine to create a functional channel. Humans possess many distinct VGKC genes, and the resulting channels exhibit specialized properties and display rich tissue-type and cellular-compartment specificity. Each α-subunit of voltage-gated channels contains six transmembrane segments (S1–S6) linked by extracellular and intracellular loops ( Fig. 98.3 ). The S5–S6 loop penetrates deep into the central part of the channel and lines the pore. The S4 segment contains positively charged amino acids and acts as the voltage sensor. These channels serve many functions, most notably to establish the resting membrane potential and to repolarize cells following an action potential. A unique class of potassium channel, the inwardly rectifying potassium channel, is homologous to the S5–S6 segments of the VGKC. Because the voltage-sensing S4 domain is absent, voltage dependence results from a voltage-dependent blockade by magnesium and polyamines rather than from the movement of the positively charged S4 domain in response to membrane depolarization.
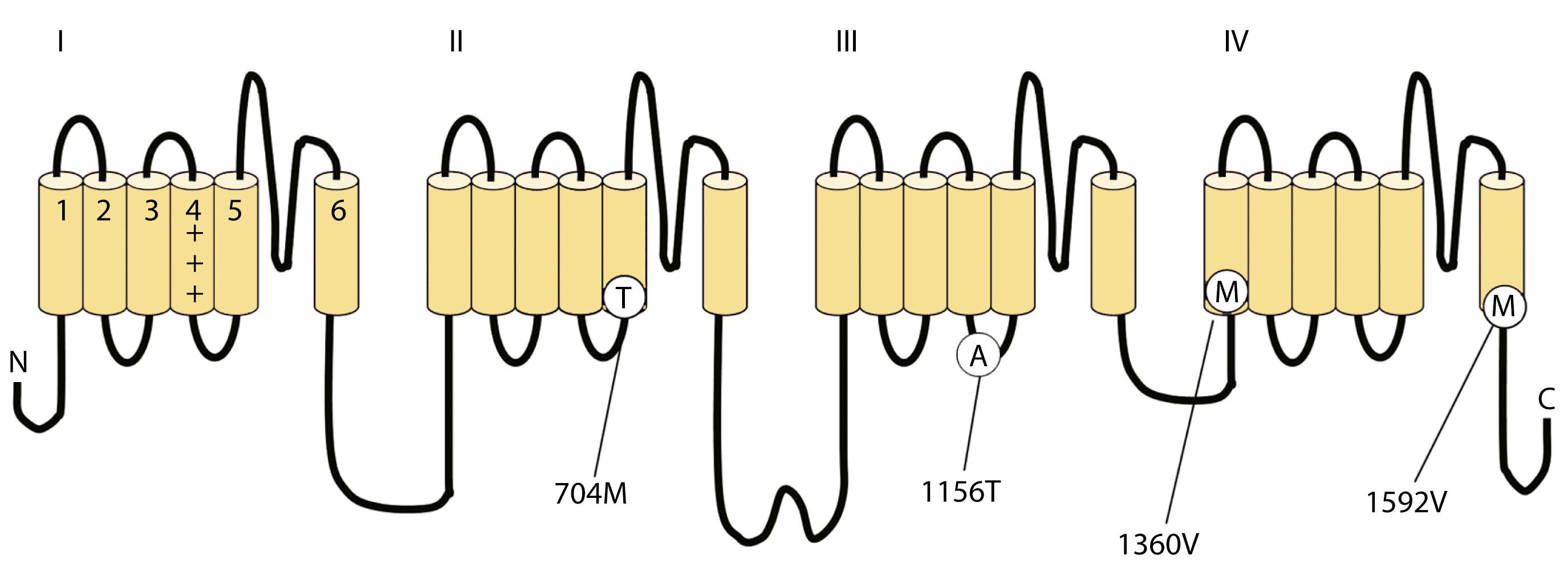
Voltage-gated sodium and calcium channels are highly homologous and share homology with VGKCs, from which they evolved. The α-subunits contain four highly homologous domains in tandem within a single transcript (DI–DIV; Fig. 98.4 ). Each domain resembles a VGKC α-subunit, with six transmembrane segments, as described earlier. Sodium and calcium channels differ in several regards, despite their many similarities. The amino acid sequence forming the selectivity filter and the modulatory auxiliary subunits are different. The sodium channel is composed of an α- and a β-subunit, and the calcium channel is composed of a pore-forming α 1 -subunit, an intracellular β-subunit, a membrane-spanning γ-subunit, and a membrane-anchoring α 2 δ-subunit. Sodium channels mediate fast depolarization and underlie the action potential, whereas voltage-gated calcium channels (VGCCs) mediate neurotransmitter release and allow the calcium influx that leads to second messenger effects.
Ligand-gated ion channels activate on binding with their respective agonists. GABA A , glycine, and nicotinic acetylcholine receptors (nAChRs) are examples of ligand-gated ion channels with known disease-causing mutations. Although distinguished by their ligand binding and ion permeability, channels gated by GABA, glycine, and acetylcholine share several structural similarities. Five intrinsic membrane subunits assemble to form hetero- or homopentamers. Each subunit contains four transmembrane domains (M1–M4), the second of which lines the pore and determines ionic selectivity ( Fig. 98.5 ). Subunits contributing to nAChRs at the neuromuscular junction differ from those expressed in the central nervous system, explaining why the mutation of one gene may cause seizures without affecting neuromuscular transmission, or vice versa. Binding of acetylcholine opens the channel, which conducts monovalent cations (Na + and K + ) with little or no selectivity, and some are additionally permeable to calcium. Channel activation results in membrane depolarization and excitation of the postsynaptic neuron or muscle fiber.
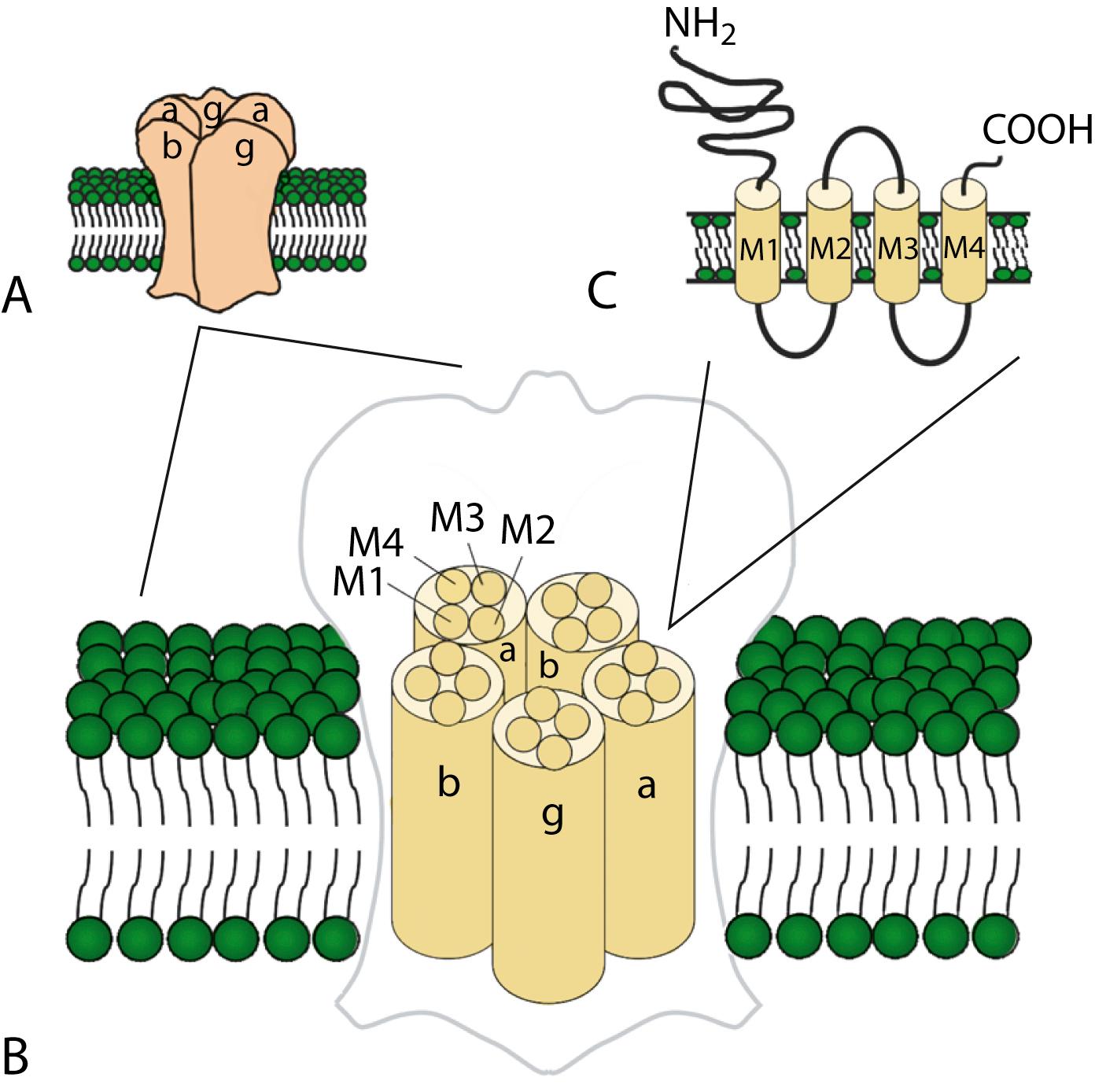
The GABA A and glycine receptors belong to the nAChR superfamily and similarly consist of five subunits. GABA A receptors include α-, β-, and either γ- or δ-subunits. The predominant glycine receptor is a heteropentamer of three α-subunits and two β-subunits. In either case, agonist binding opens the channel and allows the flux of chloride (Cl − ) into the cell, generally causing hyperpolarization and decreased excitability. Therefore, both GABA and glycine mediate inhibitory synaptic transmission.
Channelopathies encompass a variety of diseases in multiple systems, including neurological, cardiac, endocrine, and kidney disorders, as summarized in Table 98.1 . In the nervous system, most channelopathies are characterized by episodic attacks. Phenotypically, it ranges from muscle disease and peripheral neuropathy to episodic movement disorders, epilepsy, and migraine headache. Patients experience recurrent “attacks” throughout life, usually with complete resolution between attacks. Although there are strikingly diverse presentations, depending on the expression pattern of the gene, this group of disorders shares many similarities. First, as mentioned earlier, they are episodic with various frequencies of attacks. Second, these disorders have triggers or precipitating factors such as stress, sleep deprivation, and certain dietary factors that often precede the onset of an attack. Third, these disorders typically have a similar natural history, with an onset in childhood to young adulthood, worsening through adolescence and young-adult life, and improvement in middle- and late-adult life.
Skeletal muscle channelopathies are characterized by periodic paralyses and nondystrophic myotonia. These disorders include familial hypokalemic periodic paralysis (hypoKPP), hyperkalemic periodic paralysis (hyperKPP), PMC, myotonia congenita, PAM, and Andersen-Tawil syndrome (ATS). The patients with these disorders present with episodic muscle weakness, lasting minutes to hours, typically with full recovery between episodes. With recurrent episodes, one can develop permanent and fixed weakness later in life. Inheritance is autosomal dominant, with high penetrance, though for some mutations, there can be sex-dependent penetrance. Usually, there are triggers, as mentioned earlier, including stress, alcohol, recent illness, exercise followed by rest, fasting, or glucose-rich meals. Weakness can be regional or generalized, while bulbar and respiratory involvement is rare. Carbonic anhydrase inhibitors such as acetazolamide can be used to reduce the frequency of attacks of weakness. Sodium channel blockers like mexiletine are sometimes effective in reducing myotonic symptoms and signs (see Table 98.2 ).
The patients may describe myotonia as muscle “stiffness” or “cramping.” Myotonia can be found clinically, and subclinical myotonia can be detected using electromyography (EMG). Myotonia is enhanced muscle excitability that leads to sustained bursts of discharges. Due to hyperexcitability of the muscle, one experiences involuntary contraction in said muscle, resulting from the inability to relax after forceful voluntary contraction. This can be demonstrated in the exam room, asking the patient to make a tight fist or forceful eye closure, and it is called “action myotonia.” Percussion myotonia is another supporting exam finding of muscle channelopathies. When a muscle, such as the gastrocnemius muscle, is tapped with a reflex hammer, a persistent dimpling of the muscle can be seen. Myotonia is a nonspecific sign found in several other diseases, including myotonic dystrophy 1, myotonic dystrophy 2 (proximal myotonic myopathy), myotonia congenita, PMC, and hyperKPP. The “paramyotonia” in paramyotonia congenital describes the ability to demonstrate worsening of myotonic stiffness with repeated muscle contractions and is best seen in the orbicularis oculi. This can be also be precipitated by muscle cooling (i.e., a temperature-sensitive phenotype). PMC is one disorder in this group with a distinct clinical finding of paramyotonia, commonly demonstrated with repeated forceful eye closure, leading to increasing myotonia of the orbicularis oculi.
Clinical and subclinical myotonia can be demonstrated using EMG, which shows sustained bursts of muscle after-discharges that persist following voluntary contraction or occur in response to insertion of an EMG needle. It produces a characteristic “dive-bomber” discharge, with waxing and waning discharges (20–80 Hz).
In addition to distinct myotonic discharges using EMG, nerve conduction studies (NCS), in particular the short and long exercise tests (SET and LET), can be further utilized to narrow down the differential diagnosis ( ). The compound motor action potentials (CMAPs) are recorded at a basline and every 10 seconds after 10 seconds of short isometric exercise up to 60 seconds. This 60 second set of CMAP recording is repeated twice, with a resting period of 1 minute between each set. It is noteworthy that maintaining a warm temperature is important, as cooling may change the pattern with decreased CMAP amplitude yet increased CMAP duration. The LET is performed by isometric exercise for 5 minutes. CMAPs are recorded every minute during the exercise, after exercise, and every 5 minutes after exercise, for 40–45 minutes.
The pattern of decrement and increment of CMAP amplitude over time are helpful to identify distinct subgroups of mutations causing periodic paralysis. Fournier et al. (2004) analyzed the patterns after short and long exercises and categorized muscle channelopathies into 5 groups, as summarized in Table 98.4 .
| HypoKPP | HyperKPP | PMC | MC | PAM | |
|---|---|---|---|---|---|
| Age at onset | 2nd decade | 1st decade | Infancy | 1st decade | 1st–2nd decade |
| Duration of attack | Hours–days | Minutes–hours | Hours | Minutes–hours | N/A |
| Severity of attack | Moderate–severe | Mild–moderate | Mild–moderate | Mild–moderate | |
| Triggers | Postexercise, CHO load | Postexercise, fasting, K load | Cold, postexercise | Rest | K |
| Myotonia | Absent | Present | Present | Present | Present |
| Serum K + | Usually low | Normal or high | Variable | Normal | Normal |
| Progressive weakness | Some patients | Some patients | Absent | Present in Becker myotonia | Absent |
| Treatment for weakness | CAI | CAI | CAI | N/A | N/A |
| Treatment for myotonia | N/A | Mexiletine | Mexiletine | Mexiletine, phenytoin | CAI |
| Use | Prophylactic agent for some channelopathies (see text). |
| Mechanism | Inhibits carbonic anhydrase. |
| Dosing | Adults: start 125 mg daily, titrating as needed up to a maximum daily dose of 1000–1500 mg, divided bid–qid. An extended release formulation is available. Children: consult a pharmacist. |
| Side effects | Taste changes (especially for carbonated drinks), fatigue, paresthesias, metabolic acidosis, blurred vision, myelosuppression, nephrolithiasis, etc. (Increased dietary citrate might be recommended to compensate for decreased urinary citrate observed during acetazolamide therapy.) |
| Monitoring | Check electrolytes, BUN, creatinine, and CBC at baseline and periodically throughout therapy. |
| Metabolism | None; excreted unchanged by kidneys. |
| Fournier Pattern | Short Exercise Test | Long Exercise Test | Clinical Phenotype |
|---|---|---|---|
| I | Postexercise amplitude decrement that worsens with each trial | Postexercise amplitude decrement that does not return to baseline over 40 minutes | Paramyotonia congenita (T1313M or R1448C sodium) |
| II | Postexercise amplitude decrement that improves with each trials | No postexercise amplitude change or small transient decrement | Myotonia congenita (chloride channel mutation) |
| III | No postexercise amplitude change | No postexercise amplitude change | Other forms of myotonia (G1306A, 1693T sodium) |
| IV | Postexercise amplitude increment that increases with each trial | Transient postexercise amplitude increment followed by late continuous decrement over 40 minutes | HyperKPP (T704M sodium) |
| V | No postexercise amplitude change | Late continuous postexercise amplitude decrement over 40 minutes | HypoKPP-1 (R528H calcium) |
The prevalence of hypoKPP is approximately 1 per 100,000. Episodes of limb weakness accompanied by hypokalemia usually begin during adolescence. Attacks usually occur in the morning and are often triggered either by the ingestion of a carbohydrate load and high salt intake the previous night or by rest following strenuous exercise. Generalized muscle weakness and reduced or absent tendon reflexes are characteristic. Heralding the weakness may be sensory changes, fatigue, or a feeling of heaviness or aching in the legs or back. During paralysis, level of consciousness and sensation are preserved. Paralysis either spares the facial and respiratory muscles or causes only mild weakness, making medical intervention rarely necessary. The frequency, length, and severity of attacks vary. Although attacks may occur several times a week, they more often occur at intervals of weeks or months. Attack duration varies from minutes to days, typically lasting several hours. Occasionally the attacks are sufficiently brief to cause difficulty in documenting the accompanying hypokalemia. Patients usually recover full strength, although mild weakness may persist for several days or (more rarely) be permanent. A progressive permanent myopathy with mild proximal weakness may develop later in life, although it is rare.
There are largely two genes that are responsible for hypoKPP. HypoKPP1 is up to 70% of cases, and it is caused by mutations in the CACNA1S gene encoding the α 1 -subunit of the dihydropyridine-sensitive L-type voltage-gated skeletal muscle calcium channel, Ca v 1.1, on chromosome 1q32.1 ( ).
Ca v 1.1 is the slow-inactivating, L-type calcium channel and can be blocked by 1,4-dihydropyridine (e.g., amlodipine and nifedipine), phenylalkamines (e.g., verapamil), and benzothiazepines (e.g., diltiazem; ). This channel functions as the voltage sensor of the ryanodine receptor and plays an important role in excitation–contraction coupling in skeletal muscle. Four mutations in the S4 segments alter voltage sensitivity. Two mutations, involving arginine-to-histidine substitutions within the highly conserved S4 segments of DII and DIV (Arg-528-His and Arg-1239-His), account for most cases. The others involve arginine-to-glycine substitutions at the same locations.
HypoKPP2 is caused by a mutation in SCN4A gene on chromosome 17q23.3 encoding the pore-forming α-subunit of the skeletal muscle voltage-gated sodium channel, Na v 1.4. Approximately 10%–20% of families with hypoKPP have this mutation. This is the same channel implicated in hyperKPP and other disorders described later (see Fig. 98.1 ). Evidence suggests that this sodium channel–associated syndrome is phenotypically different from the more common CACNA1S form. A proposed separate clinical entity, hypoKPP2, may be distinguishable from hypoKPP1 associated with CACNA1S by the presence of myalgias following paralytic attacks, and the presence of tubular aggregates instead of vacuoles in the muscle biopsy. In some patients, acetazolamide worsens symptoms ( ). In a large retrospective series, hypoKPP2 was associated with an older age of onset and shorter duration of attacks than classical hypoKPP1 ( ).
Whether involving SCN4A or CACNA1S , virtually all mutations causing hypoKPP involve an S4 voltage-sensor domain. In the case of the sodium channel, these mutations allow a leak current to pass through the “gating pore” at resting membrane potentials, bypassing the central channel pore and leading to inappropriate muscle fiber depolarization and consequent channel inactivation and action potential failure ( ). Speculation exists that this phenomenon may also occur in mutated VGCCs.
An accurate medical history is essential for the diagnosis because observation of attacks is unusual, and patients are often normal between attacks. Characteristic features of hypoKPP that distinguish it from hyperKPP are that paralytic attacks are less frequent, longer lasting, precipitated by a carbohydrate load, and often begin during sleep (see Table 98.2 ). Potassium concentrations are usually low during an attack, less than 3.0 mM, although concentrations less than 2 mM should suggest a secondary form of periodic paralysis. Electrocardiogram (ECG) changes such as increased PR and QT intervals, T-wave flattening, and prominent U waves suggest an underlying hypokalemia. Provocative testing can be dangerous and is not routine. Test performance requires a hospitalized setting with continuous cardiac monitoring and should be performed only in patients without cardiac or renal disease. After giving an oral glucose load (2–5 g/kg up to a maximum of 100 g) with or without subcutaneous insulin (0.1 U/kg), one performs serial examinations of strength while monitoring serum glucose and potassium concentrations. Myotonia is not found in hypoKPP, either clinically or with EMG. EMG may reveal membrane irritability with myopathic changes but often is normal. Short and long exercise tests reveal Fournier pattern V, with decrement CMAPs following the long exercise test without significant change in the SET. Creatine kinase (CK) can be normal but may be elevated. Potassium between attacks is normal. Muscle histology reveals nonrimmed vacuoles within muscle fibers in biopsies in hypoKPP1 or tubular aggregates in hypoKPP2. Genetic testing should render muscle biopsy and provocative testing obsolete for diagnosis.
Thyrotoxic periodic paralysis (TPP) may be clinically indistinguishable from hypoKPP, except that it is not familial and serum potassium levels are often lower than in familial hypoKPP (<2.5). TPP is more common in Asian adults and in men more than women. Some cases may be associated with a mutation in KCNJ18 , the gene encoding a novel inwardly rectifying potassium channel ( ). All patients with hypoKPP require screening for hyperthyroidism, as the treatment—correction of the thyroid disorder—differs from that outlined for familial hypoKPP. Treatment of hypothyroidism would prevent further attacks of weakness. Beta-blockade is often effective in reducing the frequency and severity of the paralytic attacks ( ). One needs to exclude other secondary forms of hypokalemic paralysis when serum potassium concentrations remain low between attacks. Renal, adrenal, and gastrointestinal causes of persistent hypokalemia are common, and thiazide diuretic use and licorice (glycyrrhizic acid) intoxication must also be considered.
An effective holistic approach to treatment includes lifestyle modifications and acute and chronic pharmacological intervention. Dietary modification to avoid high carbohydrate loads and refraining from excessive exertion help prevent attacks. Prophylactic use of acetazolamide ( Table 98.3 ) decreases the frequency and severity of attacks. Dichlorphenamide is another carbonic anhydrase inhibitor that effectively prevents attacks, as demonstrated in a randomized clinical trial ( ) where the average dose was 100 mg daily. More potent than acetazolamide, dichlorphenamide may be useful when the efficacy of the former begins to fail. A clinician may keep in mind that hypoKPP2 caused by SCAN4A has less benefit from carbonic anhydrase inhibitors compared to patients with hypoKPP1 ( ). Potassium-sparing diuretics (e.g., loop diuretics) or angiotensin-converting enzyme inhibitors may be of prophylactic benefit in patients with normal renal function in whom other more conservative measures are insufficient. Many believe that reducing the frequency or severity of paralytic attacks provides protection against the development of myopathy based on anecdotal experience, although this has never been formally tested in a controlled trial. Inhibition of Na, K, Cl cotransporter (NKCLC), particularly with bumetanide (which is a NCLC blocker) is currently in clinical trial.
During an acute attack, the preferred method of treatment is oral potassium chloride given at 0.5–1.0 mEq/kg, not exceeding 200 mEq in a 24-hour period. If a patient is unable to take oral potassium (e.g., arrhythmia due to hypokalemia or airway compromise due to altered mental status), then intravenous potassium (KCl bolus 0.05–0.1 mEq/kg or 20–40 mEq/L of KCl in 5% mannitol) is indicated. Cardiac monitoring is important during the administration of potassium.
Characteristic of this disorder is episodic weakness precipitated by hyperkalemia. Although the weakness is generally milder than in hypoKPP, it can be sufficiently severe in hyperKPP to cause flaccid quadriparesis. As in hypoKPP, respiratory and ocular muscles are unaffected and consciousness is preserved. The frequency of attacks varies from several per day to several per year. Attacks are usually brief, lasting 15–60 minutes, but may last up to days. Unlike hypoKPP, myotonia is present between attacks. Onset is usually in infancy or childhood, and characteristic attacks occur by adolescence. Triggers include rest after vigorous exercise, foods high in potassium, fasting, stress, and fatigue. Despite its name, hyperKPP is often associated with a normal serum potassium concentration during an attack (see the following Diagnosis section). Most patients experience a subacute onset, and some describe paresthesia or a sensation of muscle tension prior to attacks. In these situations, mild exercise may abort or lessen the severity of the attack. The thigh and calf muscles are often involved. Generalized weakness is uncommon, making hyperKPP distinct from hypoKPP. Mild weakness may persist afterward, and the later development of a progressive myopathy is common.
HyperKPP is an autosomal dominant disorder, with high penetrance, and there are some sporadic cases. The disorder links to SCN4A , the same gene responsible for a minority of hypoKPP cases. Among several identified missense mutations, four account for about two-thirds of cases (see Fig. 98.1 ). Functional expression of naturally occurring mutations demonstrated a decrease in the voltage threshold of channel activation or abnormally prolonged channel opening or both ( ), effectively increasing the depolarizing inward current. If sustained long enough, this would lead to inactivation of the sodium channels, transitory cellular inexcitability, and weakness.
Despite advances in defining the underlying genetic mutations, a thorough medical and family history and physical examination remain the best diagnostic tools. Serum potassium is normal between attacks and even during many attacks. Unlike hypoKPP, potassium administration may precipitate an attack. In the absence of provocative testing, the basis for diagnosis is the clinical presentation. Hyperkalemia itself can cause weakness; thus secondary causes of hyperkalemia should be considered, especially if the potassium levels are greater than 7 mEq/L. Myotonia is present in many patients between attacks, either spontaneously or after muscle percussion, and failure to produce myotonia discriminates hypoKPP from hyperKPP (see Table 98.2 ). Take care not to confuse subjective muscle stiffness with objective changes. Peaked T waves on ECG suggest hyperkalemia and are an aid to diagnosis. As in hypoKPP, serum CK concentrations are often normal but sometimes can be elevated. A potassium-loading test provokes an attack but is not usually necessary and can be dangerous.
Electrodiagnostic studies are useful for demonstrating subclinical myotonic discharges, not seen in hypoKPP. Myopathic findings such as fibrillation potentials and small polyphasic motor unit potentials occur during late stages of disease. Interictal NCS are usually normal, but the CMAPs during the attack may be reduced. The SET and LET reveal a Fournier pattern IV, ( Table 98.4 ) where the SET shows increased amplitude after the first trial, which is further increased in subsequent trials, and the LET shows a transient increase in amplitude after exercise, followed by a gradual decrement in amplitude over 40 minutes.
The goal of therapy is to abort the acute attacks and prevent future attacks. Acute attacks are often sufficiently brief and mild, so as not to require acute intervention. In more severe attacks, aim treatment at lowering extracellular potassium levels. Mild exercise or eating a high sugar load (juice or a candy bar) may suffice, as insulin drives extracellular potassium into cells. Thiazide diuretics and inhaled β-adrenergic agonists are similarly helpful, and intravenous glucose, insulin, or calcium gluconate may be used for severe weakness. To prevent attacks, a diet low in potassium and high in carbohydrates may obviate the need for prophylactic drug therapy. Patients are also advised to avoid fasting, strenuous exercise, and cold. Oral dichlorphenamide (50–150 mg/day) was useful for prophylaxis in one randomized controlled trial ( ). Acetazolamide (see Table 98.3 ) and thiazide diuretics are useful as well. Successful prophylaxis may decrease the later onset of myopathy, although direct proof of this hypothesis is lacking. Finally, myotonic symptoms are troublesome in some patients; sodium channel blockers, such as mexiletine, can be used for symptomatic management. Obtaining ECGs is advised due to QT prolongation. ( )
The characteristic features of PMC are paradoxical myotonia, cold-induced myotonia, and weakness after prolonged cold exposure. Unlike classic myotonia, which shows a warm-up phenomenon (see the later section Myotonia Congenita), patients with PMC often show exacerbation of myotonia after repeated muscle contraction. Symptoms may be recognized in infants and certainly by childhood and usually remain unchanged throughout life, although one may develop progressive permanent weakness over time. Infants may be noted to have difficulty opening their eyes after crying due to myotonia of the orbicularis oculi. Myotonia affects all skeletal muscles, although the facial muscles and muscles of the neck and hands are the most common sites of myotonia in the winter. The onset of weakness is often during the day, lasts several hours, and is exacerbated by cold, stress, and rest after exercise. Many patients are asymptomatic when warm. However, cold-induced stiffness may persist for hours even after the body warms, and percussion myotonia is present even when the patient is otherwise asymptomatic.
The cause of PMC is point mutations in the SCN4A gene on chromosome 17q. Thus, PMC is allelic to hyperKPP, PAM, and less commonly, hypoKPP. Mutations of the gene, which include substitutions at T1313 on the DIII to DIV linker and at R1448 on the DIV–S4 segment, cause defects in sodium channel deactivation and fast inactivation. The resting membrane potential rises from −80 up to −40 mV when intact muscle fibers cool. Mild depolarization results in repetitive discharges (myotonia), whereas greater depolarization results in sodium channel inactivation and muscle inexcitability (weakness).
A family history of exercise- and cold-induced stiffness or difficulty of relaxation after forceful contraction strongly supports the diagnosis of PMC. When asked to close their eyes forcefully and repeatedly, affected individuals exhibit progressive difficulty with relaxation and are eventually unable to open their eyes; this is called paramyotonia, the name derived from paradoxical reaction to exercise. Furthermore, muscle cooling may elicit an increase in myotonia, a reduction in isometric force of 50% or more, and a prolongation of the relaxation time by several seconds after muscle cooling supports the diagnosis. Serum potassium concentration may be high, low, or normal during attacks, and serum CK concentrations may be elevated 5–10 times normal. Electrodiagnostic studies are useful in establishing the diagnosis. EMG reveals fibrillation-like potentials and myotonic discharges that are accentuated by muscle percussion, needle movement, and muscle cooling. Muscle cooling elicits an initial increase in myotonia, then a progressive decrease in myotonia, followed by a decrease in CMAP amplitude that correlates respectively, with muscle stiffness and weakness. The SET commonly reveals postexercise myotonic potentials (PEMPs) after a short exercise on the motor conduction studies. CMAP amplitudes show delayed decrement after second and third trials, particularly in patients with T1313M mutation. The SET in patients with Q270K mutation shows prominent decrement with cooling. The LET shows decrement in CMAP amplitudes during and after exercise without return to its baseline. These SET and LET patterns are categorized to Fournier pattern I, which is distinctive for PMC ( Table 98.4 ). Muscle pathology shows only nonspecific changes, and biopsy is unnecessary.
Symptoms are generally mild and infrequent. Direct treatment, when required, at either myotonia or weakness or both. Sodium channel blockers such as mexiletine are effective in reducing the frequency and severity of myotonia, as shown in a randomized trial ( ). Patients with weakness often respond to agents used to treat hyperKPP (e.g., thiazides, acetazolamide). A single case report suggests the possible use of pyridostigmine ( ). Cold avoidance reduces the frequency of attacks.
Inheritance of myotonia congenita (MC) is either as an autosomal dominant (Thomsen disease) or recessive (Becker myotonia) trait. The main feature is myotonia or delayed muscle relaxation after contraction. Forceful movement abruptly initiated after several minutes of rest causes the most pronounced myotonic stiffness. The myotonia of MC displays a warm-up phenomenon in which the myotonia decreases or vanishes completely when repeating the same movement several times, in contrast to the myotonia seen in patients with PMC. Unlike PMC, cold temperature does not exacerbate the clinical or electrical myotonia or weakness ( ) The onset of Thomsen disease is often within the first decade, whereas the onset of Becker myotonia is generally at 10–14 years of age. Although myotonia can affect all skeletal muscles, it is especially prominent in the legs, where it is occasionally severe enough to impede a patient’s ability to walk or run. In rare cases, sudden noise causes sufficient generalized stiffness to make the patient fall to the ground and remain rigid for several seconds. The recessive and dominant forms share many similarities, but some clinical features help distinguish the two. In general, patients with recessive disease experience transitory bouts of weakness after periods of disuse and may develop progressive myopathy ( ); in addition, muscle hypertrophy and disease severity are greater than in the dominant form. Becker myotonia is more common than Thomsen disease. In contrast to myotonic dystrophies, affected individuals have no systemic disorders such as cardiomyopathy, cataracts, endocrinopathies, ventilatory weakness, and skeletal deformities.
Electrical instability of the sarcolemma leads to muscle stiffness by causing repetitive electric discharges of affected muscle fibers. Early in vivo studies in myotonic goats revealed greatly diminished sarcolemmal chloride conductance in affected muscle fibers. This causes a depolarization of the sarcolemmal membrane and muscle hyperexcitability. Genetic linkage analysis for both recessive and dominant forms of MC pointed to a locus on chromosome 7q, where the responsible gene, CLCN1 , encodes the major skeletal muscle chloride channel. More than 70 mutations have been identified within CLCN1 , and, interestingly, some of these mutations are recognized to cause both dominant and recessive forms ( ). Examination of the functional effects of several myotonia-causing CLCN1 mutations in heterologous expression systems reveals effects on channel gating or membrane expression levels, usually resulting in a decreased chloride conductance ( ).
On clinical exam, patients may display muscle hypertrophy, often giving patients a muscular or athletic appearance. Muscle strength and reflexes are normal, although there may be mild proximal weakness. Tapping the belly of a muscle with a percussion hammer can elicit percussion myotonia that lasts for several seconds. Action myotonia can be also demonstrated by having patients make a strong grip or closing the eyes tightly and then opening them. With repetitive contractions, one may find relaxation easier, due to a warm-up phenomenon.
Electrodiagnostic testing is also helpful. NCS may find decremental CMAP amplitudes on repetitive stimulation at 10 Hz or higher. EMG also demonstrates myotonia. The SET and LET may show transient decrease in CMAP after the first trial and no significant change in amplitudes after exercise, respectively (Fournier pattern II, Table 98.4 ). This pattern is seen more commonly in individuals with the autosomal recessive form. Greater than 20% decrement of amplitude-and-area during SET is specific for MC ( ). Biopsy is usually nonspecific, showing enlarged fibers in hypertrophied muscle, increased numbers of internalized nuclei, and decreased type 2B fibers ( Table 98.4 ).
Many patients (especially with Thomsen disease) experience only mild symptoms and do not require treatment. For those with more severe myotonia (especially with Becker myotonia), sodium channel blocking agents remain the mainstay of treatment. Mexiletine is the most commonly used and was shown to be effective in treating myotonia in a preliminary randomized placebo-controlled study ( ). Other sodium channel blockers such as tocainide, phenytoin, procainamide, and quinine exhibit variable degrees of efficacy.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here