Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Ischemia during the perioperative period demands immediate attention by the anesthesiologist.
Nitroglycerin is indicated in most cases of perioperative myocardial ischemia. Mechanisms of action include coronary vasodilation and favorable alterations in preload and afterload. Nitroglycerin is contraindicated in cases of hypotension.
Perioperative β-blockade may reduce the incidence of perioperative myocardial ischemia by several mechanisms when initiated at an appropriate time in the preoperative period. Favorable hemodynamic changes associated with β-blockade include blunting of the stress response and reduced heart rate, blood pressure, and contractility. All of these conditions improve myocardial oxygen supply-to-demand ratios.
Calcium channel blockers reduce myocardial oxygen demand by depression of contractility, reduction of heart rate, and decrease in arterial blood pressure. Calcium channel blockers are often administered in the perioperative period for long-term antianginal symptomatic control.
Mild or moderate hypertension does not represent an independent risk factor for perioperative complications, but a diagnosis of hypertension necessitates preoperative assessment for target organ damage.
Patients with poorly controlled preoperative hypertension experience more labile blood pressures in the perioperative setting with a greater potential for hypertensive and hypotensive episodes.
The signs, symptoms, and treatment of chronic heart failure are related to the neurohormonal response and underlying ventricular dysfunction.
Treatments for chronic heart failure are aimed at prolonging survival, along with relief of symptoms.
The pathophysiology, treatment, and prognosis of low cardiac output syndrome seen after cardiac surgery are different from those of chronic heart failure, with which it is sometimes compared.
Physicians must be cautious in administering antiarrhythmic drugs because their proarrhythmic effects can increase mortality for certain subgroups of patients.
Amiodarone has become a popular intravenous antiarrhythmic drug for use in the operating room and critical care areas because it has a broad range of effects for ventricular and supraventricular arrhythmias.
β-Receptor antagonists are effective but underused antiarrhythmics in the perioperative period because many arrhythmias are adrenergically mediated owing to the stress of surgery and critical illness.
Managing electrolyte abnormalities and treating underlying disease processes such as hypervolemia and myocardial ischemia are critical treatment steps before the administration of any antiarrhythmic agent.
Perioperative myocardial ischemia is an anesthetic emergency that should be treated promptly with appropriate therapy. All events of myocardial ischemia involve an alteration in the oxygen supply-to-demand balance. For the anesthetized patient with evidence of myocardial ischemia, initiation of antiischemic drug therapy is the primary intervention.
Nitroglycerin (NTG) is clinically indicated as initial therapy for most types of myocardial ischemia. During therapy with intravenous NTG, if blood pressure (BP) drops and ischemia is not relieved, the addition of phenylephrine allows coronary perfusion pressure to be maintained while allowing higher doses of NTG to be used for ischemia relief. If reflex increases in heart rate and contractility occur, combination therapy with β-adrenergic blockers may be indicated to blunt the undesired increase in heart rate. Combination therapy with nitrates and calcium channel blockers may be an effective antiischemic regimen in selected patients.
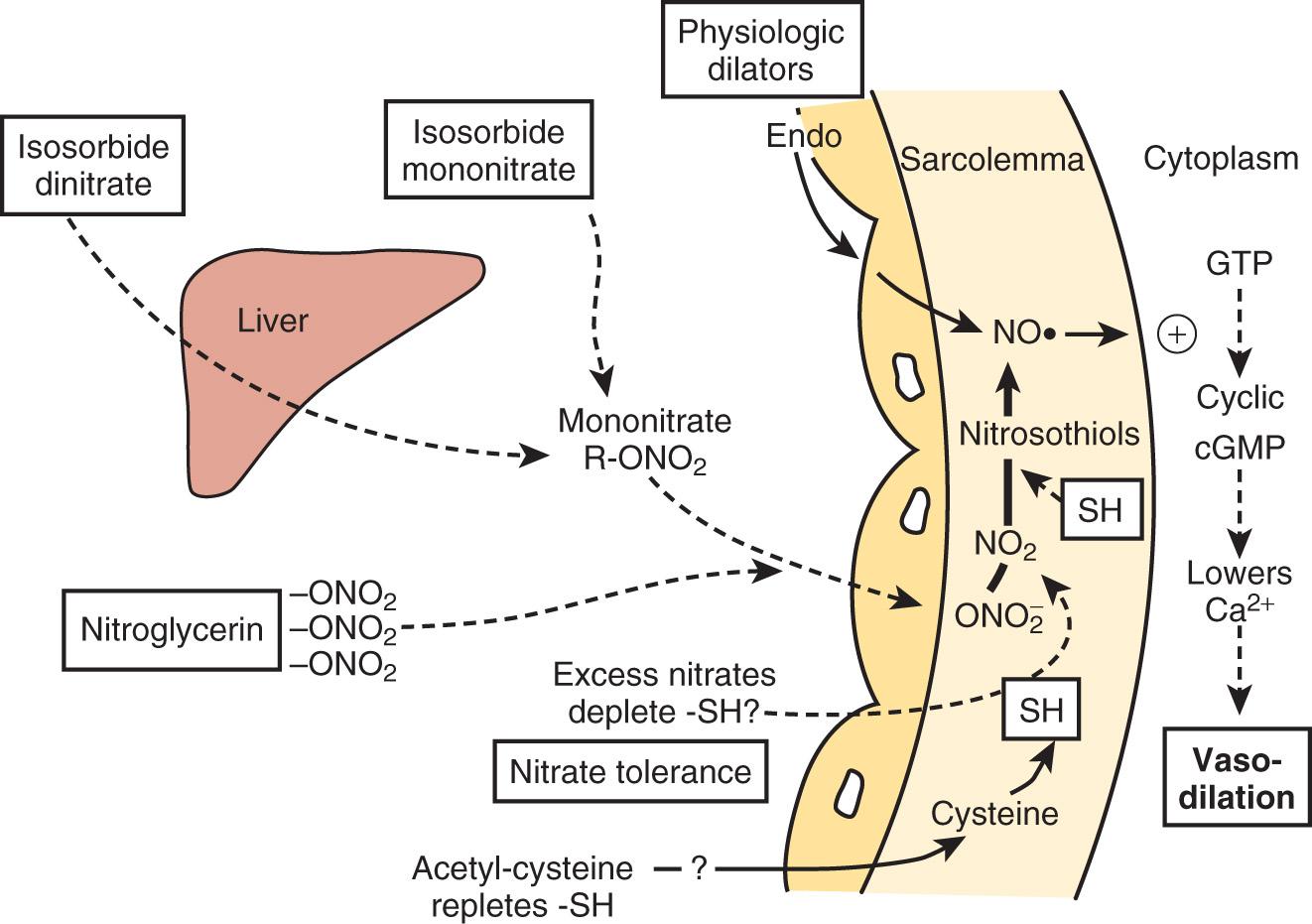
NTG enhances myocardial oxygen delivery and reduces myocardial oxygen demand. NTG is a smooth muscle relaxant that causes vasculature dilation. Nitrate-mediated vasodilation occurs with or without intact vascular endothelium. Nitrites, organic nitrites, nitroso compounds, and other nitrogen oxide–containing substances (eg, nitroprusside) enter the smooth muscle cell and are converted to reactive nitric oxide (NO) or S -nitrosothiols, which stimulate guanylate cyclase metabolism to produce cyclic guanosine monophosphate (cGMP)( Fig. 8.1 ). A cGMP-dependent protein kinase is stimulated with resultant protein phosphorylation in the smooth muscle. This leads to a dephosphorylation of the myosin light chain and smooth muscle relaxation. Vasodilation is also associated with a reduction of intracellular calcium. Sulfhydryl (SH) groups are required for formation of NO and the stimulation of guanylate cyclase. When excessive amounts of SH groups are metabolized by prolonged exposure to NTG, vascular tolerance occurs. The addition of N -acetylcysteine, an SH donor, reverses NTG tolerance. The mechanism by which NTG compounds are uniquely better venodilators, especially at lower serum concentrations, is unknown but may be related to increased uptake of NTG by veins compared with arteries.
Two important physiologic effects of NTG are systemic and regional venous dilation. Venodilation can markedly reduce venous pressure, venous return to the heart, and cardiac filling pressures. Prominent venodilation occurs at lower doses and does not increase further as the NTG dose increases. Venodilation results primarily in pooling of blood in the splanchnic capacitance system. Mesenteric blood volume increases as ventricular size, ventricular pressures, and intrapericardial pressure decrease.
NTG increases the distensibility and conductance of large arteries without changing systemic vascular resistance (SVR) at low doses. At higher doses, NTG dilates smaller arterioles and resistance vessels, reducing afterload and BP. Reductions in cardiac dimension and pressure reduce myocardial oxygen consumption (MV̇ o 2 ) and improve myocardial ischemia. NTG may preferentially reduce cardiac preload while maintaining systemic perfusion pressure, an important hemodynamic effect in myocardial ischemia. However, in hypovolemic states, higher doses of NTG may reduce systemic BP to dangerous levels. A reflex increase in heart rate may occur at arterial vasodilating doses.
NTG causes vasodilation of pulmonary arteries and veins and predictably decreases right atrial, pulmonary artery, and pulmonary capillary wedge pressures (PCWP). Pulmonary artery hypertension may be reduced by NTG in various disease states and in congenital heart disease. Renal arteries, cerebral arteries, and cutaneous vessels also dilate with NTG.
NTG has several important effects on the coronary circulation ( Box 8.1 ). It is a potent epicardial coronary artery vasodilator in normal and diseased vessels. Stenotic lesions dilate with NTG, reducing the resistance to coronary blood flow (CBF) and improving myocardial ischemia. Smaller coronary arteries may dilate relatively more than larger coronary vessels, but the degree of dilation may depend on the baseline tone of the vessel. NTG effectively reverses or prevents coronary artery vasospasm.
Epicardial coronary artery dilation: small arteries dilate proportionately more than larger arteries
Increased coronary collateral vessel diameter and enhanced collateral flow
Improved subendocardial blood flow
Dilation of coronary atherosclerotic stenoses
Initial short-lived increase in coronary blood flow; later reduction in coronary blood flow as myocardial oxygen consumption decreases
Reversal and prevention of coronary vasospasm and vasoconstriction
NTG has been available since the early 1980s as a parenteral drug with a stable shelf half-life in a 400-µg/mL solution of 5% dextrose in water (D 5 W). Blood levels are achieved instantaneously, and arterial dilating doses with resulting hypotension may quickly occur. If the volume status of the patient is unknown, initial doses of 5 to 10 µg/min are recommended. The dose necessary for relieving myocardial ischemia may vary from patient to patient, but relief is usually achieved with 75 to 150 µg/min. Arterial dilation becomes clinically apparent at doses around 150 µg/min. Drug offset after discontinuation of an infusion is rapid (2–5 minutes).
NTG remains a first-line agent for the treatment of myocardial ischemia. Special care must be taken in patients with signs of hypovolemia or hypotension because vasodilating effects of the drug may worsen the clinical condition ( Box 8.2 ). The 2014 American College of Cardiology/American Heart Association (ACC/AHA) guidelines address the prophylactic intraoperative use of NTG and state there is no benefit in preventing myocardial ischemia and cardiac morbidity in high-risk patients undergoing noncardiac surgery and that NTG use could be harmful.
Patients with ongoing ischemic discomfort should receive sublingual nitroglycerin (0.4 mg) every 5 minutes for a total of three doses, after which an assessment should be made about the need for intravenous nitroglycerin (level of evidence [LOE] C).
Intravenous nitroglycerin is indicated for relief of ongoing ischemic discomfort, control of hypertension, or management of pulmonary congestion (LOE C).
Nitrates should not be administered to patients with systolic blood pressure less than 90 mm Hg or greater than or equal to 30 mm Hg below baseline, severe bradycardia (<50 beats/min), tachycardia (>100 beats/min), or suspected right ventricular infarction (LOE C).
Nitrates should not be administered to patients who have received a phosphodiesterase inhibitor for erectile dysfunction within the last 24 hours (48 hours for tadalafil) (LOE B).
ACC , American College of Cardiology; AHA , American Heart Association; STEMI , ST-segment elevation myocardial infarction.
β-Adrenergic blockers have multiple favorable effects in treating the ischemic heart during anesthesia ( Box 8.3 ). β-Adrenergic blockers reduce oxygen consumption by decreasing heart rate, BP, and myocardial contractility. Heart rate reduction increases diastolic CBF. Increased collateral blood flow and redistribution of blood to ischemic areas may occur with β-blockers. β-Blockers should be started early in ischemic patients in the absence of contraindications. If hemodynamics prevent concomitant NTG and β-blocker use, β-blockers should receive precedence. Many patients at high risk for perioperative cardiac morbidity should be started on β-blockers before surgery and continued for up to 30 days after surgery. Adequate time in initiating β-blocker therapy should be allowed to adjust dosing before surgical procedures.
Reductions in myocardial oxygen consumption
Improvements in coronary blood flow
Prolonged diastolic perfusion period
Improved collateral flow
Increased flow to ischemic areas
Overall improvement in the supply-to-demand ratio
Stabilization of cellular membranes
Improved oxygen dissociation from hemoglobin
Inhibition of platelet aggregation
Reduced mortality rate after myocardial infarction
Many studies have shown that perioperative administration of β-adrenergic blockers reduces mortality and morbidity when given to patients at high risk for coronary artery disease (CAD) who must undergo noncardiac surgery. These data suggest that intermediate- and high-risk patients undergoing noncardiac surgery should receive perioperative β-adrenergic blockade to reduce postoperative cardiac mortality and morbidity.
Propranolol has an equal affinity for β 1 - and β 2 -receptors, lacks intrinsic sympathomimetic activity (ISA), and has no β-adrenergic receptor activity ( Table 8.1 ). It is the most lipid-soluble β-blocker and has the most central nervous system (CNS) side effects. Because the rate of first-pass liver metabolism is very high (90%), it requires much higher oral doses than intravenous doses for pharmacodynamic effect. The usual intravenous dose of propranolol initially is 0.5 to 1.0 mg titrated to effect. A titrated dose resulting in maximal pharmacologic serum levels is 0.1 mg/kg.
| Drug | Selectivity | Partial Agonist Activity | Usual Dose for Angina |
|---|---|---|---|
| Propranolol | None | No | 20–80 mg bid |
| Metoprolol | β 1 | No | 50–200 mg bid |
| Atenolol | β 1 | No | 50–200 mg/d |
| Nadolol | None | No | 40–80 mg/d |
| Timolol | None | No | 10 mg bid |
| Acebutolol | β 1 | Yes | 200–600 mg bid |
| Betaxolol | β 1 | No | 10–20 mg/d |
| Bisoprolol | β 1 | No | 10 mg/d |
| Esmolol (infusion) | β 1 | No | 50–300 µg⋅kg −1 ⋅min −1 |
| Labetalol a | None | Yes | 200–600 mg tid |
| Pindolol | None | Yes | 2.5–7.5 mg tid |
Metoprolol was the first clinically used cardioselective β-blocker. Its affinity for β 1 -receptors is 30 times higher than its affinity for β 2 -receptors. As with any cardioselective β-blocker, higher serum levels may result in greater incidence of β 2 -blocking effects. Metoprolol is administered intravenously in 1- to 2-mg doses, titrated to effect. The potency of metoprolol is approximately one-half that of propranolol. Maximal β-blocker effect is achieved with 0.2 mg/kg given intravenously.
The chemical structure of esmolol is similar to that of metoprolol and propranolol, except that it has a methyl ester group in the para-position of the phenyl ring, making it susceptible to rapid hydrolysis by red blood cell esterases (ie, 9-minute half-life). Esmolol is not metabolized by plasma cholinesterase. Hydrolysis results in an acid metabolite and methanol with clinically insignificant levels. Ninety percent of the drug is eliminated in the form of the acid metabolite, normally within 24 hours. A loading dose of 500 µg/kg given intravenously followed by a 50 to 300 µg/kg per minute infusion reaches steady-state concentrations within 5 minutes. Without the loading dose, steady-state concentrations are reached in 30 minutes.
Esmolol is cardioselective, blocking primarily β 1 -receptors. It lacks ISA and membrane-stabilizing effects and is mildly lipid soluble. Esmolol produced significant reductions in BP, heart rate, and the cardiac index after a loading dose of 500 µg/kg and an infusion of 300 µg/kg per minute in patients with CAD, and the effects were completely reversed 30 minutes after discontinuation of the infusion. Initial therapy during anesthesia may require significant reductions in the loading and infusion doses.
Hypotension is a common side effect of intravenous esmolol. The incidence of hypotension was higher with esmolol (36%) than with propranolol (6%) at equal therapeutic end points. The cardioselective drugs may cause more hypotension because of β 1 -induced myocardial depression and the failure to block β 2 peripheral vasodilation. Therefore administering a test dose of 20 mg IV is a good clinical practice.
Labetalol is an equal mixture of four stereoisomers with various α- and β-blocking properties. Labetalol provides selective α 1 -receptor blockade and nonselective β 1 - and β 2 -blockade. The potency of β-adrenergic blockade is 5- to 10-fold greater than α 1 -adrenergic blockade. Labetalol has partial β 2 -agonist effects that promote vasodilation. It is moderately lipid soluble and is completely absorbed after oral administration. First-pass hepatic metabolism is significant, with production of inactive metabolites. Renal excretion of the unchanged drug is minimal. Elimination half-life is approximately 6 hours.
In contrast to other β-blockers, labetalol should be considered a peripheral vasodilator that does not cause a reflex tachycardia. BP and systolic vascular resistance decrease after an intravenous dose. Stroke volume (SV) and cardiac output (CO) remain unchanged, with the heart rate decreasing slightly. The reduction in BP is dose related, and acutely hypertensive patients usually respond within 3 to 5 minutes after a bolus dose of 100 to 250 µg/kg. However, the more critically ill or anesthetized patients should have their BP titrated, beginning with 5- to 10-mg intravenous increments. The BP reduction may last as long as 6 hours after intravenous dosing.
β-Adrenergic blockers are first-line agents in the treatment of myocardial ischemia. These agents effectively reduce myocardial work and oxygen demand. Although perioperative β-blockers may decrease perioperative cardiovascular events in noncardiac surgery, the benefit may come at an increased short-term risk for severe complications, including stroke and death if started too close to the time of surgery.
Calcium channel blockers reduce myocardial oxygen demands by depression of contractility, heart rate, and arterial BP. Myocardial oxygen supply may be improved by dilation of coronary and collateral vessels. In an acute ischemic situation, calcium channel blockers (ie, verapamil and diltiazem) may be used for rate control when β-blockers cannot be used.
The most important effects of calcium channel blockers may be the treatment of variant angina. These drugs can attenuate ergonovine-induced coronary vasoconstriction in patients with variant angina, suggesting protection by coronary dilation. Most episodes of silent myocardial ischemia, which may account for 70% of all transient ischemic episodes, are not related to increases in myocardial oxygen demands (ie, heart rate and BP); instead, intermittent obstruction of coronary flow is likely caused by coronary vasoconstriction or spasm. All calcium channel blockers are effective at reversing coronary spasm, reducing ischemic episodes, and reducing NTG consumption in patients with variant or Prinzmetal angina.
Combinations of NTG and calcium channel blockers, which also effectively relieve and possibly prevent coronary spasm, are rational therapy for variant angina. β-Blockers may aggravate anginal episodes in some patients with vasospastic angina and should be used with caution. Preservation of CBF with calcium channel blockers is a significant difference from the predominant β-blocker antiischemic effects of reducing MV̇ o 2 .
Systemic hemodynamic effects of calcium channel blockers in vivo represent a complex interaction among myocardial depression, vasodilation, and reflex activation of the autonomic nervous system ( Table 8.2 ).
| Characteristic | Amlodipine | Diltiazem | Nifedipine | Verapamil |
|---|---|---|---|---|
| Heart rate | ↑/0 | ↓ | ↑/0 | ↓ |
| Sinoatrial node conduction | 0 | ↓↓ | 0 | ↓ |
| Atrioventricular node conduction | 0 | ↓ | 0 | ↓ |
| Myocardial contractility | ↓/0 | ↓ | ↓/0 | ↓↓ |
| Neurohormonal activation | ↑/0 | ↑ | ↑ | ↑ |
| Vascular dilatation | ↑↑ | ↑ | ↑↑ | ↑ |
| Coronary flow | ↑ | ↑ | ↑ | ↑ |
Nifedipine, like all dihydropyridines (DHPs), is a potent arterial dilator with few venodilating effects. Reflex activation of the sympathetic nervous system (SNS) may increase heart rate. The intrinsic negative inotropic effect of nifedipine is offset by potent arterial dilation, which lowers BP and increases CO in patients. DHPs are excellent antihypertensive agents because of their arterial vasodilatory effects. Antianginal effects result from reduced myocardial oxygen requirements owing to the afterload-reducing effect and to coronary vascular dilation resulting in improved myocardial oxygen delivery.
Verapamil is a less potent arterial dilator than the DHPs and results in less reflex sympathetic activation. In vivo, verapamil usually results in moderate vasodilation without significant changes in heart rate, CO, or SV. Verapamil can significantly depress myocardial function in patients with preexisting ventricular dysfunction.
Diltiazem is a less potent vasodilator and has fewer negative inotropic effects compared with verapamil. Clinical studies reveal reductions in SVR and BP, with increases in CO, pulmonary arterial wedge pressure, and ejection fraction (EF).
Coronary artery dilation occurs with the calcium channel blockers, along with increases in total CBF. Nifedipine is the most potent coronary vasodilator, especially in epicardial vessels, which are prone to coronary vasospasm. Diltiazem is effective in blocking coronary artery vasoconstriction caused by a variety of agents, including α-agonists, serotonin, prostaglandin, and acetylcholine.
Nicardipine is a DHP agent with vascular selectivity for coronary and cerebrovascular beds. Nicardipine may be the most potent overall relaxant of vascular smooth muscle among the DHPs. Peak plasma levels are reached 1 hour after oral administration, with bioavailability of 35%. Plasma half-life is approximately 8 to 9 hours. Although the drug undergoes extensive hepatic metabolism, with less than 1% of the drug excreted renally, greater renal elimination occurs in some patients. Plasma levels may increase in patients with renal failure, and reduction of the dose is recommended in these patients.
Clevidipine is a DHP agent with a unique chemical structure that renders it inactive by cleavage of an ester linkage by nonspecific esterases in the blood and in tissues. This unique property renders it extremely short acting, similar to other drugs (eg, esmolol) that are metabolized through this pathway. Its initial phase half-life is 1 minute, with 90% of the drug eliminated. Its clinical effects are fully reversed in 5 to 15 minutes for most patients after discontinuing the infusion.
Clevidipine is a potent arterial vasodilator whose primary use is as a parenteral antihypertensive agent. A reflexive tachycardia may be seen with its use in healthy volunteers and patients with essential hypertension that, combined with possible hypotension, would limit its role in treating ongoing myocardial ischemia. In studies looking at perioperative and postoperative cardiac surgical patients, clevidipine was effective in decreasing mean arterial pressure but did not affect heart rate or filling pressures.
The structure of verapamil is similar to that of papaverine. Verapamil exhibits significant first-pass hepatic metabolism, with a bioavailability of only 10% to 20%. One hepatic metabolite, norverapamil, is active and has a potency approximately 20% of that of verapamil. Peak plasma levels are reached within 30 minutes. Bioavailability markedly increases in hepatic insufficiency, mandating reduced doses. Intravenous verapamil achieves hemodynamic and dromotropic effects within minutes, peaking at 15 minutes and lasting up to 6 hours. Accumulation of the drug occurs with prolonged half-life during long-term oral administration.
After oral dosing, the bioavailability of diltiazem is greater than that of verapamil, varying between 25% and 50%. Peak plasma concentration is achieved between 30 and 60 minutes, and the elimination half-life is 2 to 6 hours. Protein binding is approximately 80%. As with verapamil, hepatic clearance is flow dependent, and major hepatic metabolism occurs, with metabolites having 40% of the clinical activity of diltiazem. Hepatic disease may require decreased dosing, whereas renal failure does not affect dosing.
Systemic hypertension, long recognized as a leading cause of cardiovascular morbidity and mortality, accounts for enormous health-related expenditures. Almost one-fourth of the US population has hypertensive vascular disease, but 30% of these individuals are unaware of their condition, and another 30% to 50% are inadequately treated. On a worldwide basis, almost 1 billion individuals are hypertensive. Hypertension management comprises the most common reason underlying adult visits to primary care physicians, and antihypertensive drugs are the most prescribed medication class. Despite the asymptomatic nature of hypertensive disease, with symptom onset delayed 20 to 30 years after development of systemic hypertension, substantial and incontrovertible evidence demonstrates a direct association between systemic hypertension and increased morbidity and mortality. The World Health Organization (WHO) estimates that hypertension underlies one in eight deaths worldwide, making elevated BP the third leading cause of mortality.
Hypertension is the single most treatable risk factor for myocardial infarction (MI), stroke, peripheral vascular disease, congestive heart failure (CHF), renal failure, and aortic dissection. In prospective, randomized trials, over the course of adult lifetimes, successful treatment of hypertension has been associated with a 35% to 40% reduction in the incidence of stroke, 50% reduction in CHF, and 25% reduction in MIs. Improved treatment of hypertension has been credited with the major reductions in stroke and cardiovascular mortality occurring in the United States during the past 30 years.
The Eighth Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC8) provided significant modifications to prior recommendations for the management of high BP. In contrast to prior guidelines, JNC8 recommendations are derived from evidence-based guidelines drawn only from randomized, controlled trials (RCTs). Specific recommendations emanating from the new guidelines include lifestyle interventions and pharmacologic treatment as needed to attain systolic BPs less than 150 mm Hg and diastolic BPs less than 90 mm Hg for adults 60 years of age and older. For younger patients and those with diabetes or chronic kidney disease, treatment goals include systolic BPs less than 140 mm Hg and diastolic BPs less than 90 mm Hg.
Although antihypertensive drug therapy is widely regarded as essential for BPs greater than 150/90 mm Hg, evidence suggests benefits to more aggressive BP reduction for certain patient subsets. The association between systemic BP and cardiovascular risk has been described as a J curve, with progressive cardiovascular risk reductions accompanying BP reductions until a critical threshold, after which the potential for myocardial ischemia and other organ injury increases. Risk for cardiovascular disease appears to increase at BPs greater than 115/75 mm Hg, with a doubling in risk associated with each 20/10-mm Hg increment in systemic pressure.
Almost 80 distinct medications are marketed for the treatment of hypertension. Combined therapy with two or more classes of antihypertensive medications is often needed to achieve treatment goals. Although the specific drug selected for initial therapy is deemed less important than in the past, recognition that specific antihypertensive drug classes alleviate end-organ damage beyond that associated with reductions in systemic BP has led to targeted selection of antihypertensive drug combinations on the basis of coexisting risk factors such as recent MI, chronic renal insufficiency, or diabetes.
Severe hypertension may be characterized as a hypertensive emergency, with target organ injury (eg, myocardial ischemia, stroke, pulmonary edema), or as hypertensive urgency, with severe elevations in BP not yet associated with target organ damage. Specific BPs associated with these conditions prove somewhat arbitrary, but BPs exceeding 220/125 mm Hg pose an immediate risk for life-threatening end-organ damage. A hypertensive emergency necessitates immediate therapeutic intervention, most often with intravenous antihypertensive therapy and invasive arterial BP monitoring. In the most extreme cases of malignant hypertension , severe elevations in BP may be associated with retinal hemorrhages, papilledema, and evidence of encephalopathy, which may include headache, vomiting, seizure, and coma. Progressive renal failure and cardiac decompensation may characterize the most severe hypertensive urgencies.
Sodium nitroprusside, long favored as a parenteral treatment for hypertensive urgencies in intraoperative settings ( Table 8.3 ), acts as an NO donor to induce arterial and venous dilation. A rapid physiologic response and relatively predictable titratable effect prove useful for intraoperative settings. However, the potency of sodium nitroprusside and the potential for prolonged administration to be associated with cyanide or thiocyanate toxicity have provided an opportunity for newer parenteral antihypertensive drugs.
| Drug | Dose | Onset of Action | Duration of Action | Adverse Effects b | Special Indications |
|---|---|---|---|---|---|
| Nicardipine hydrochloride | 5–15 mg/h IV | 5–10 min | 15–30 min, may exceed 4 h | Tachycardia, headache, flushing, local phlebitis | Most hypertensive emergencies except acute heart failure; caution with coronary ischemia |
| Clevidipine | 1–2 mg/h IV | 2–4 min | 5–15 min | Headache, nausea, vomiting, soy and egg allergy cross-reactivities | Most hypertensive emergencies except severe aortic stenosis |
| Sodium nitroprusside | 0.25–10 µg/kg per minute as IV infusion c | Immediate | 1–2 min | Nausea, vomiting, muscle twitching, sweating, thiocyanate and cyanide intoxication | Most hypertensive emergencies; caution with high intracranial pressure or azotemia |
| Fenoldopam mesylate | 0.1–0.3 µg/kg per min IV infusion | <5 min | 30 min | Tachycardia, headache, nausea, flushing | Most hypertensive emergencies; caution with glaucoma |
| Nitroglycerin | 5–100 µg/min as IV infusion | 2–5 min | 5–10 min | Headache, vomiting, methemoglobinemia, tolerance with prolonged use | Coronary ischemia |
| Enalaprilat | 1.25–5 mg every 6 h IV | 15–30 min | 6–12 h | Precipitous fall in pressure in high-renin states; variable response | Acute left ventricular failure; avoid in acute myocardial infarction |
| Hydralazine hydrochloride | 10–20 mg IV | 10–20 min IV | 1–4 h IV | Tachycardia, flushing, headache, vomiting, aggravation of angina | Eclampsia |
| 10–40 mg IM | 20–30 min IM | 4–6 h IM | |||
| Adrenergic Inhibitors | |||||
| Labetalol hydrochloride | 20–80 mg IV bolus every 10 min | 5–10 min | 3–6 h | Vomiting, scalp tingling, dizziness, nausea, heart block, orthostatic hypotension, bronchoconstriction | Most hypertensive emergencies except acute heart failure |
| 0.5–2.0 mg/min IV infusion | |||||
| Esmolol hydrochloride | 250–500 µg/kg per minute IV bolus, then 50–100 µg/kg per minute by infusion; may repeat bolus after 5 min or increase infusion to 300 µg/min | 1–2 min | 10–30 min | Hypotension, nausea, asthma, first-degree heart block, heart failure | Aortic dissection, perioperative |
| Phentolamine | 5–15 mg IV bolus | 1–2 min | 10–30 min | Tachycardia, flushing, headache | Catecholamine excess |
a Doses may vary from those in the Physicians Desk Reference (PDR).
Nicardipine and clevidipine, parenteral DHP calcium channel blockers, have proved particularly applicable for hypertensive urgencies in perioperative settings. Although less potent and predictable than sodium nitroprusside, NTG, another NO donor, may be preferable in the setting of myocardial ischemia or after coronary artery bypass graft (CABG) surgery.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here