Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Autism spectrum disorder (ASD) is a neurobiologic disorder with onset in early childhood; it is characterized by impaired social communication and interaction accompanied by restricted and repetitive behaviors.
With definitions that vary dependent on agencies, schools, and clinicians, there is often confusion surrounding the diagnosis of ASD. This is the term used for all children who were, in previous editions of the Diagnostic and Statistical Manual of Mental Disorders (DSM), divided into autism, Asperger syndrome, and pervasive developmental disorder not otherwise specified. The reliability and validity of the separation of these diagnoses were shown to be directly correlated to the diagnosing facility and not a distinct set of diagnostic criteria or biomarkers, and, therefore, the diagnoses were collated under the umbrella of autism spectrum disorder (DSM-5).
Several criteria must be met to enable the diagnosis of ASD beyond difficulties in socialization ( Tables 32.1 and 32.2 ). It should be noted that although these features must be present early in the developmental period, there is no age cutoff for making a diagnosis.
| A. |
|
|
|
|
|
|
|
| B. |
|
|
|
|
|
|
|
|
|
| C. |
|
| D. |
|
| E. |
|
|
|
|
|
The diagnostic criteria in the DSM-5 focus on symptoms in two primary domains. A thorough history of previous behaviors that meet criteria is sufficient for being present early in development. As with all symptoms in the DSM-5, these behaviors must cause significant impairment to be a disorder. Finally, the symptoms cannot be better explained by intellectual disability (intellectual developmental disorder) or global developmental delay (see Table 32.1E ) or other disorders ( Table 32.3 ).
|
|
|
|
|
|
|
|
|
|
|
There are several modifiers and levels that help clinicians better communicate about the abilities and deficits of an individual with ASD. The modifiers include those for accompanying intellectual impairment, accompanying language impairment, if the diagnosis is associated with a known medical or genetic condition, and if it is associated with another neurodevelopmental, mental, or behavioral disorder. There is also a modifier if catatonia is present. The level system adds a way to indicate how much support an individual needs, from level 1, where minimal support is required and an untrained observer may not quickly notice they have ASD, to level 3, where very substantial support is required due to their symptoms ( Table 32.4 ). Common clinical features are noted in Table 32.5 .
| Severity Level | Social Communication | Restricted, Repetitive Behaviors |
|---|---|---|
| Level 3 “Requiring very substantial support” |
Severe deficits in verbal and nonverbal social communication skills cause severe impairments in functioning, very limited initiation of social interactions, and minimal response to social overtures from others For example, a person with few words of intelligible speech who rarely initiates interaction and, when they do, they make unusual approaches to meet needs only and respond to only very direct social approaches. | Inflexibility of behavior, extreme difficulty coping with change, or other restricted/repetitive behaviors markedly interfere with functioning in all spheres. Great distress/difficulty changing focus or action. |
| Level 2 “Requiring substantial support” |
Marked deficits in verbal and nonverbal social communication skills; social impairments apparent even with supports in place; limited initiation of social interactions; and reduced or abnormal responses to social overtures from others. For example, a person who speaks simple sentences, whose interaction is limited to narrow special interests, and who has markedly odd nonverbal communication. | Inflexibility of behavior, difficulty coping with change, or other restricted/repetitive behaviors appear frequently enough to be obvious to the casual observer and interfere with functioning in a variety of contexts. Distress and/or difficulty changing focus or action. |
| Level 1 “Requiring support” |
Without supports in place, deficits in social communication cause noticeable impairments. Difficulty initiating social interactions, and clear examples of atypical or unsuccessful responses to social overtures of others. May appear to have decreased interest in social interactions. For example , a person who is able to speak in full sentences and engages in communication but whose to-and-fro conversation with others fails, and whose attempts to make friends are odd and typically unsuccessful. | Inflexibility of behavior causes significant interference with functioning in one or more contexts. Difficulty switching between activities. Problems of organization and planning hamper independence. |
| Social Interaction and Reciprocal Communication Behaviors | |
|---|---|
Spoken Language
Responding to Others
Interacting with Others
|
Eye Contact, Pointing, and Other Gestures
Ideas and Imagination
Unusual or Restricted Interests and/or Rigid and Repetitive Behaviors
|
A thorough physical examination can help to guide next steps. Children with ASD frequently react adversely to the focused attention of an examination, and, as a result, indirect techniques such as observation and examination by an experienced clinician are required. A dysmorphology evaluation (see Chapter 29 ) can aid with identifying comorbid syndromes and guide genetic testing. Facial asymmetry, multiple hair whorls, and prominent forehead have been described as occurring more often in patients with autism. There are reports of rapid head growth in the 1st year of life in some individuals. Specific organ system malformations in individuals with ASD behaviors should raise the suspicion of a congenital malformation syndrome associated with autistic-like behavior ( Table 32.6 ).
Chromosome Deletions
Chromosome Duplications
Epilepsy Encephalopathies, Epilepsy
|
Idd Cdkl5 (Rett-Like) Regression, Others
|
During the initial physical work-up, it is important to rule out other major medical concerns that can mimic autism (see Table 32.3 ). A formal hearing screen is essential; relying on newborn hearing screening or screening undertaken at school is not sufficient when considering autism. Due to the child’s difficulty in participating in the test, it is important to test in a manner that does not involve child response, even if they are older. If a vision screening has not been performed adequately, this should also be included. A Woods lamp examination of the skin should be conducted to rule out neurocutaneous disorders such as tuberous sclerosis and neurofibromatosis type 1.
All children should be screened for autism at the 18- and 24-month well-child visits. There are screening checklists that parents can complete prior to the visit, which are then reviewed by the physician. The Modified Checklist for Autism in Toddlers, Revised (M-CHAT-R) ( https://m-chat.org/en-us/page/take-m-chat-test/online ) is one of the most widely used at ages 18–30 months. It is free, electronic for easy scoring, and available in many languages. The Social Communication Questionnaire is available for children ages 4 years and older with a mental age above 2 years and contains 40 yes-or-no questions for parents with a simple cutoff score. These and other screening tools are not diagnostic but are meant to screen for parental concerns that could lead to a work-up for ASD.
Autism spectrum disorder is a clinical diagnosis that can be made by qualified clinicians familiar with the diagnostic criteria, nuances of diagnosis, and experience in the field. However, there is an important role for neuropsychologic testing in unclear cases and this is often required for the child to obtain services. The gold standard test is the Autism Diagnostic Observation Schedule (ADOS), which is available for individuals 12 months of age through adulthood and requires about an hour to administer. The person administering the exam must have special training and certification in the ADOS. The test has good specificity and sensitivity in laboratory settings but has been met with some criticism in community settings. The Autism Diagnostic Inventory–Revised (ADI-R) can be used with those children who have a mental age of 2 years and older, requires a few hours, and is given by a trained administrator to the caregiver. Its biggest limitation is that it does not include any observation or interaction with the child. The Childhood Autism Rating Scale–2 (CARS-2) is a 10-minute observation tool used by trained neuropsychologists and psychologic testers to observe the child and uses an unscored parent questionnaire.
A neuropsychologic examination of a child with developmental delays or suspected ASD should go beyond testing for autism to determine the child’s cognition and adaptive functioning, which is of utmost importance in determining what types of support they need. If the patient is able to participate, intelligence testing is available for verbal and nonverbal children and adults of all ages. This helps screen for intellectual disability but also examines what areas of intelligence are strengths and weaknesses for the child and will be used as building blocks for targeted interventions. Adaptive functioning testing, such as the Vineland Adaptive Behavior Scales, can help to identify how the child is functioning overall, which may be the most helpful part of the diagnosis to use in intervention. Often individuals with autism are not functioning where expected for chronological or even cognitive age . Very intelligent individuals with ASD can struggle with toilet training, food preparation, and other activities of daily living. Neuropsychologic assessment is also useful in planning school and home interventions.
There are many cognitive and communication difficulties that can appear to be ASD (see Table 32.3 ). These can also be co-occurring with ASD and further complicate the overall picture ( Table 32.7 ).
| Comorbidity | Individuals with Autism Affected | Comments |
|---|---|---|
| Developmental Disorders | ||
| Intellectual disability | ∼45% | Prevalence estimate is affected by the diagnostic boundary and definition of intelligence (e.g., whether verbal ability is used as a criterion). |
| In individuals, discrepant performance between subtests is common. | ||
| Language disorders | Variable | In the DSM-IV, language delay was a defining feature of autism (autistic disorder), but is no longer included in the DSM-5. |
| An autism-specific language profile (separate from language disorders) exists, but with substantial interindividual variability. | ||
| Attention-deficit/ hyperactivity disorder | 28–44% | In the DSM-IV, not diagnosed when occurring in individuals with autism, but no longer so in the DSM-5. |
| Tic disorders | 14–38% | Approximately 6–5% have Tourette syndrome. |
| Motor abnormality | ≤79% | See Table 32.2 . |
| General Medical Disorders | ||
| Epilepsy | 8–35% | Increased frequency in individuals with intellectual disability or genetic syndromes. |
| Two peaks of onset: early childhood and adolescence. | ||
| Increases risk of poor outcome. | ||
| Gastrointestinal problems | 9–70% | Common symptoms include chronic constipation, abdominal pain, chronic diarrhea, and gastroesophageal reflux. |
| Associated disorders include gastritis, esophagus, gastroesophageal reflux disease, inflammatory bowel disease, celiac disease, Crohn disease, and colitis. | ||
| Immune dysregulation | ≤38% | Associated with allergic and autoimmune disorders. |
| Genetic disorders | 10–20% | Collectively called syndromic autism. |
| Examples include fragile X syndrome (21–50% of individuals affected have autism). Rett syndrome (most have autistic features but with profiles different from idiopathic autism), tuberous sclerosis complex (24–60%), Down syndrome (5–39%), phenylketonuria (5–20%). | ||
| CHARGE syndrome ∗ (15–50%), Angelman syndrome (50–81%), Timothy syndrome (60–70%), and Joubert syndrome (-40%). | ||
| Sleep disorders | 50–60% | Insomnia is the most common. |
| Psychiatric Disorders | ||
| Anxiety | 42–56% | Common across all age groups. |
| Most common are social anxiety disorder (13–29% of individuals with autism) and generalized anxiety disorder (13–22%). | ||
| High-functioning individuals are more susceptible (or symptoms are more detectable). | ||
| Depression | 12–70% | Common in adults, less common in children. |
| High-functioning adults who are less socially impaired are more susceptible (or symptoms are more detectable). | ||
| Obsessive-compulsive disorder (OCD) | 7–24% | Shares the repetitive behavior domain with autism that could cut across nosologic categories. |
| Important to distinguish between repetitive behaviors that do not involve intrusive, anxiety-causing thoughts or obsessions (part of autism) and those that do (and are part of OCD). | ||
| Psychotic disorders | 12–17% | Mainly in adults. |
| Most commonly recurrent hallucinosis. | ||
| High frequency of autism-like features (even a diagnosis of ASD) preceding adult-onset (52%) and childhood-onset schizophrenia (30–50%). | ||
| Substance use disorders | ≤16% | Potentially because individual is using substances as self-medication to relieve anxiety. |
| Oppositional defiant disorder | 16–28% | Oppositional behaviors could be a manifestation of anxiety, resistance to change, stubborn belief in the correctness of own point of view, difficulty seeing another’s point of view, poor awareness of the effect of own behavior on others, or no interest in social compliance. |
| Eating disorders | 4–5% | Could be a misdiagnosis of autism, particularly in females, because both involve rigid behavior, inflexible cognition, self-focus, and focus on details. |
| Personality Disorders † | ||
| Paranoid personality disorder | 0–19% | Could be secondary to difficulty understanding others’ intentions and negative interpersonal experiences. |
| Schizoid personality disorder | 21–26% | Partly overlapping diagnostic criteria. |
| Schizotypal personality disorder | 2–13% | Some overlapping criteria, especially those shared with schizoid personality disorder. |
| Borderline personality disorder | 0–9% | Could have similarity in behaviors (e.g., difficulties in interpersonal relationships, misattributing hostile intentions, problems with affect regulation), which requires careful differential diagnosis. |
| Could be a misdiagnosis of autism, particularly in females. | ||
| Obsessive compulsive personality disorder | 19–22% | Partly overlapping diagnostic criteria. |
| Avoidant personality disorder | 13–25% | Could be secondary to repeated failure in social experiences. |
| Behavioral Disorders | ||
| Aggressive behaviors | ≤68% | Often directed toward caregivers rather than noncaregivers. |
| Could be a result of empathy difficulties, anxiety, sensory overload, disruption of routines, and difficulties with communication. | ||
| Self-injurious behaviors | ≤50% | Associated with impulsivity and hyperactivity, negative affect, and lower levels of ability and speech. |
| Could signal frustration in individuals with reduced communication, as well as anxiety, sensory overload, or disruption of routines. | ||
| Could also become a repetitive habit. | ||
| Could cause tissue damage and need for restraint. | ||
| Pica | ∼36% | More likely in individuals with intellectual disability. |
| Could be a result of a lack of social conformity to cultural categories of what is deemed edible, or sensory exploration, or both. | ||
| Suicidal ideation or attempt | 11–14% | Risks increase with concurrent depression and behavioral problems, and after being teased or bullied. |
∗ Coloboma of the eye, heart defects; atresia of the choanae; retardation of growth or development, or both; genital or urinary abnormalities, or both; and ear abnormalities and deafness.
Communication disorders are difficult to recognize and require careful observation and standardized testing. Communication disorders often do not become apparent until the expectations of the environment exceed the individual’s abilities. For severe disorders this could be very early in life, whereas for a mild disorder it could be several years into school when a problem is identified.
Receptive language disorder is a communication disorder where the process of receiving and comprehending language is abnormal. Expressive language disorder is difficulty with the production of vocal, gestural, and verbal signals. They do not have to be impaired together because these correlate with separate regions of the brain. Having either one, or both, presents significant challenges. These may present when children have difficulties in school that seem different from attention-deficit/hyperactivity disorder (ADHD) or learning disabilities.
In receptive language disorders , individuals have difficulty processing verbal input. They may appear as though they have not been listening. In conversation, it is frequently interpreted that they do not understand what others were saying. When disciplined through verbal command, they appear disobedient and repeat the behaviors. Yet when expressive language is preserved, they can converse, express themselves, and give others direction without apparent concern. This often causes difficulty in school and can have educators and parents blaming the child for not listening or paying attention. They can appear socially awkward or not able to fully participate in peer interactions when understanding is difficult.
When expressive language disorder is present, the individual has a difficult time expressing themselves but can understand completely what is being said to them. This can lead to frustration in the child who cannot express questions well to the teacher, cannot explain how they complete a task, or cannot give a presentation to the class. These issues can make the child appear socially awkward as well and lead to embarrassment and social isolation. Not being able to express yourself verbally can also extend to explaining emotions and desires. Behavioral issues can result from the frustration and inability to express those to others.
Pragmatic language disorder is a very narrow spectrum disorder for those individuals who have difficulty in the social rules of verbal and nonverbal communication without any of the other symptoms of ASD such as repetitive behaviors, narrow ranges of interest, or insistence on routines. This diagnosis may apply when social difficulties cannot be better explained by intellectual developmental disorder (IDD) or inattention. Although many children with ADHD have deficits in nonverbal communication, this is not considered pragmatic language disorder because the cause is not being able to attend long enough to observe details of others’ behavior.
The association of ASD and IDD is often difficult to separate. Approximately 50% or half of people with ASD have IDD. The communication difficulties that many children with ASD have can make formal cognitive assessment challenging, but more so it can make the results inaccurate. How a person with ASD performs on an IQ test may be more representative of their level of function to participate than their actual cognitive ability. In nonautistic individuals IQ test subsets are usually within a narrow range, but in autism they can be scattered over a wide range, indicating strength in some areas and weakness in others. No IQ pattern has been identified to be diagnostic in ASD. Since the diagnosis of IDD no longer requires an IQ score for severity, overall functional cognition can be used in assessment, but participation and effort are required for accurate results. It is important not to assume overall cognitive strength or functional ability from a single skill. While completing calculus in their sophomore year of high school, an individual may struggle with reading comprehension on a grade school level, be unable to complete their daily hygiene without direct support, not know what money is, or be unable to prepare food.
IDD is not just cognitive intelligence but also function or adaptive reasoning. The changes in the DSM-5 well articulate the conceptual, social, and practical domains that are affected in IDD. An individual with IDD may socialize more appropriately for their cognitive age than their chronological age. If one is to consider a diagnosis of ASD in addition to IDD, the socialization must be different from that expected for the developmental age with the inclusion of stereotyped or repetitive behaviors, restricted areas of interest, inflexibility of routines, and unusual sensory input that are not expected with that developmental age. This is even more difficult in those with severe and profound IDD who already have very limited social understanding, self-stimulating behaviors, and stereotypies. The diagnosis of ASD should only be added if the symptoms of ASD are above and beyond what can be seen in this population.
The addition of the IDD diagnosis to ASD can be very difficult when the autism symptoms are impairing daily functioning and participation in standardized testing. The use of alternate communication tools and applied behavior analysis (ABA) therapy can help to identify cognitive strengths and functioning that are limited in their expression by autism.
There is a wide spectrum of disorders that have autistic-like behavior ( Tables 32.6, 32.8, and 32.9 and Fig. 32.1 ). There is no definitive monogenetic etiology for most patients with classic ASD; rather, ASD represents diverse pathoetiologic pathways, which overlap in a similar behavioral phenotype. It remains the current recommendation that all children with a developmental disorder such as ASD or IDD undergo genetic testing if a cause of the disorder is not otherwise known. One in five children with a neurodevelopmental disability has an identifiable genetic risk factor and that number is increasing rapidly with the application of advanced genetic diagnostic tools. The importance of reaching a unifying diagnosis can impact numerous areas including screening for comorbidities, helping families understand and accept the diagnosis and natural history, and making important reproductive decisions in addition to the potential development of specific therapy. As individuals with specific genetic diagnoses increase, parent support groups allow for sharing of common experience that can help families manage and cope, especially for their neurotypical siblings.
|
| Gene or Genomic Region | Associated Syndrome | Key Features |
|---|---|---|
| 15q11-q13 | Chromosome 15q11-q13 duplication syndrome | Autism, intellectual disability, ataxia seizures, developmental delays, and behavioral problems Note: Deletion of this region is associated with Angelman syndrome/Prader-Willi syndrome |
| Chromosome 21 | Down syndrome | Distinct facial dysmorphisms, intellectual disability, congenital anomalies, and medical comorbidities |
| 22q13.3/SHANK3 | Phelan-McDermid syndrome | Neonatal hypotonia, global developmental delay, absent or severely delayed speech, autistic behavior, and minor dysmorphic features |
| FMR1 | Fragile X syndrome | Moderate to severe intellectual disability, macro-orchidism, and distinct facial features (long face, large ears, and prominent jaw) |
| TSC1/2 | Tuberous sclerosis | Multisystem disorder characterized by hamartomas (brain, heart, lungs, kidneys, and skin) |
| PTEN | PTEN -related disorders | Hamartoma syndromes and malignancies (breast, thyroid, and endometrial) Macrocephaly and ASD have been reported in children with PTEN mutations |
| MECP2 | MECP2 -related disorders | Severe neurodevelopmental disorder characterized by arrest of development between 6 and 18 mo of age, regression of skills, loss of speech, stereotypic hand movements, microcephaly, seizures, and intellectual disability |
| CDKL5 | CDKL5 -related disorders | X-linked dominant condition characterized by early onset of seizures, severe global developmental delay, and postnatal microcephaly Other features include subtle dysmorphic facial features, sleep disturbances, gastrointestinal problems, stereotypic hand movements, and intellectual disability |
| FOXG1 | FOXG1 -related disorders | Severe neurodevelopmental disorder with features of classic Rett syndrome but earlier onset in the first months of life |
| MEF2C | MEF2C -related disorders | Severe neurodevelopmental disorder characterized by intellectual disability, epilepsy, and stereotypic movements |
| CASK | CASK -related disorders | Characterized by a distinct malformation phenotype in females involving postnatal microcephaly and pontine and cerebellar hypoplasia, developmental delay, growth retardation, and eye abnormalities |
| SCN2A | SCN2A -related disorders | Autosomal dominant seizure disorder characterized by infantile onset or refractory seizures |
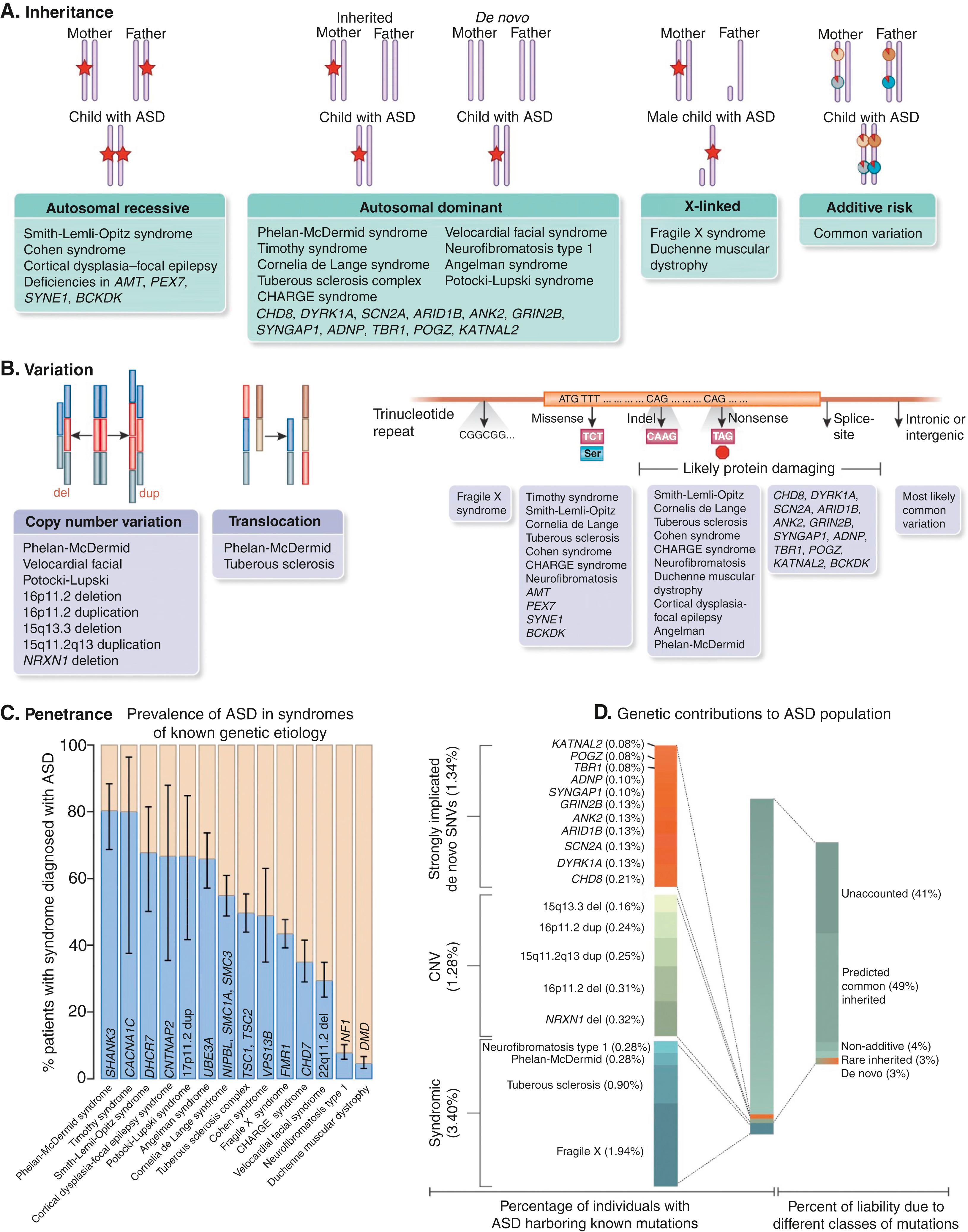
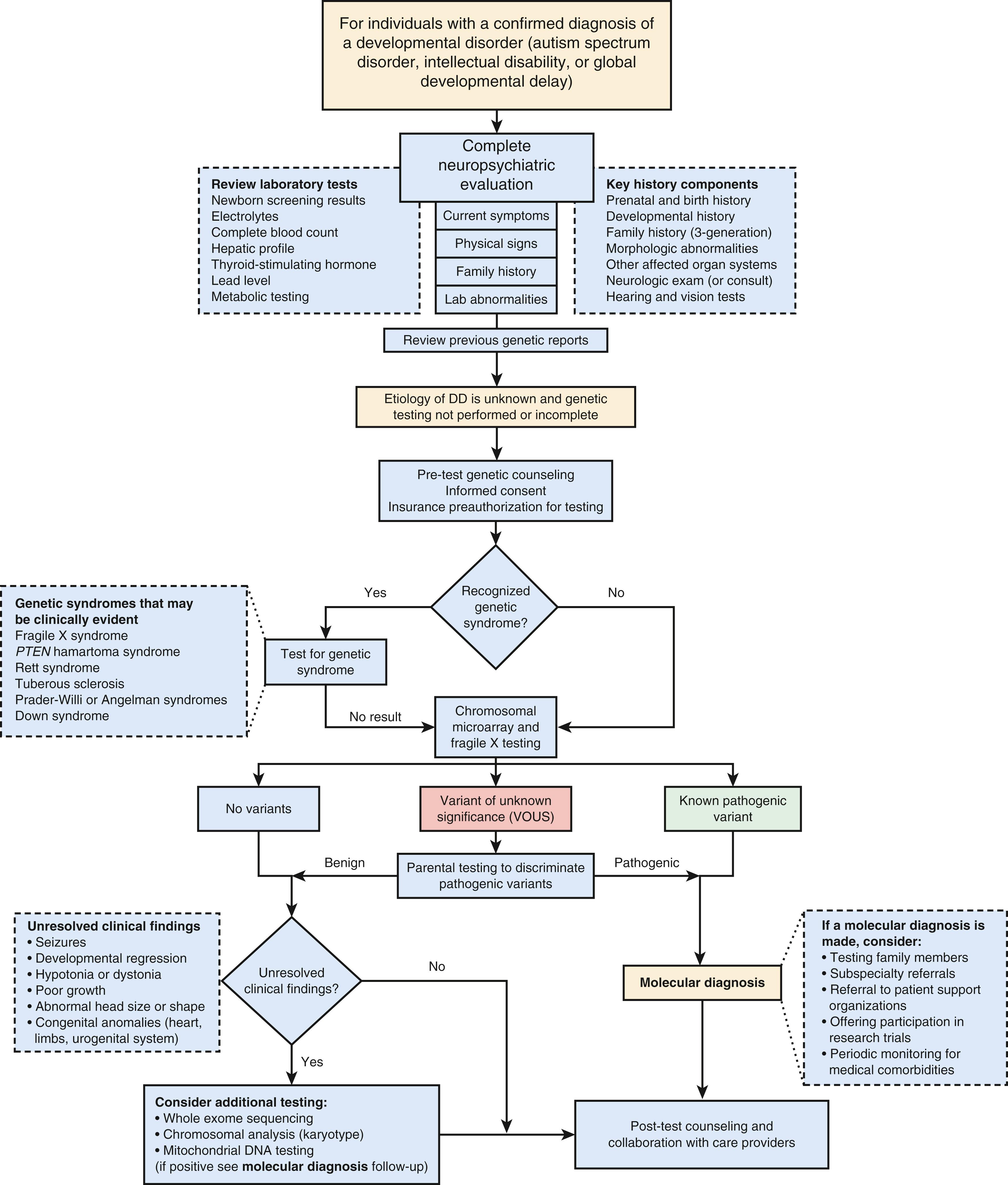
The current standard of care supports genetic testing in the form of a chromosomal microarray for all individuals with IDD or an ASD diagnosis. Fragile X testing should be considered if there is a family history of IDD, premature ovarian failure, or fragile X–associated tremor/ataxia syndrome (FXTAS) in an elderly male relative. With the advance of next-generation sequencing technologies, large IDD/ASD gene panels are available that screen hundreds to thousands of genes simultaneously. These gene panels are curated by the laboratories and are updated frequently based on published gene associations. It is important to be familiar with what test is being offered or to work with a genetic professional when ordering such testing. The current literature supports using exome sequencing as standard of care for first-tier testing, but this test has variable insurance coverage.
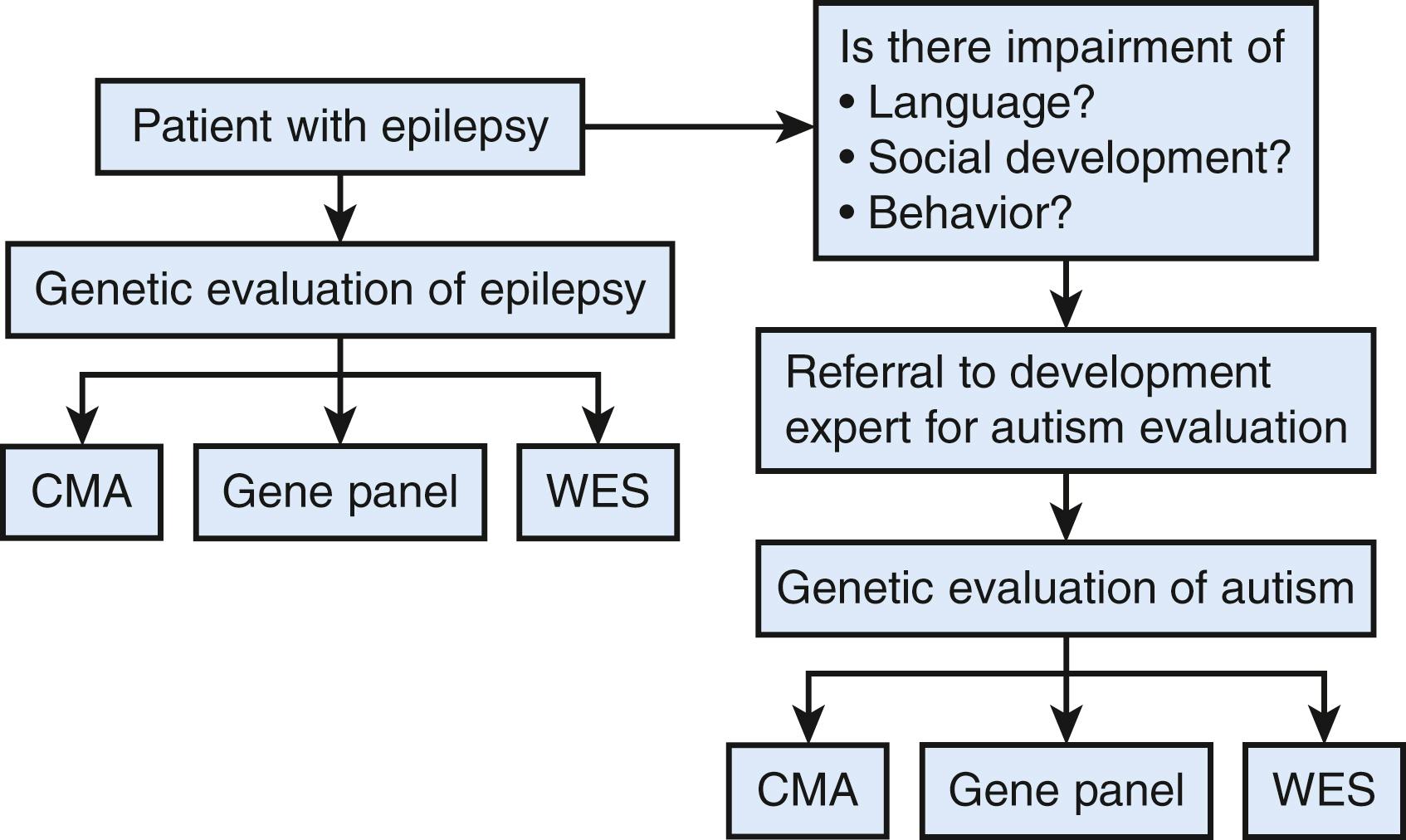
The diagnostic testing algorithm detailed in Figure 32.2 provides a comprehensive approach for diagnostic consideration. For patients with seizures see Figure 32.3 . Although epilepsy is more common in children with ASD (onset in infancy or adolescence), there are epilepsy syndromes that have autistic-like behaviors (see Table 32.9 ).
Fragile X syndrome is the leading identifiable monogenetic cause of ASD. IDD is observed in about 85% of males and 20–30% of females with fragile X syndrome; ASD is seen is 30–43% of males and 16–20% of females. Cognitive deficits are seen in executive functioning, working memory, short-term memory, and processing. Hyperarousal to sensory stimuli of all types is common. Individuals with fragile X syndrome have an increased risk of psychiatric co-occurring illnesses such as ADHD and anxiety.
The physical manifestations can be very subtle in the young child showing midface hypoplasia with sunken eyes, large-cupped ears, arched palate, and macro-orchidism. Medical features commonly include mitral valve prolapse, seizures, migraines, neuropathy, otitis media, strabismus, joint laxity, ataxia, fibromyalgia, and gastrointestinal (GI) problems. Sleep disturbances are common and can be accompanied by sleep apnea and restless leg syndrome; affected individuals have difficulty with sleep latency and maintaining sleep.
Pathogenic variants in MECP2 cause a spectrum of disorders that include Rett syndrome, PPM-X syndrome, MECP2 duplication syndrome, and MECP2 -related severe neonatal encephalopathy. Rett syndrome affects females and classically presents with a history of normal psychomotor development for the first 6–18 months of life. Development then stagnates for a period before rapid regression in motor and language skills before long-term stability is reached. During the period of regression, stereotypic hand movements replace purposeful hand movements and are accompanied by behavioral issues with screaming and inconsolable crying. There can be panic-like attacks as well as bruxism and episodic apnea and/or hyperpnea, which make caring for an affected child difficult for parents. Autistic-like features can be present although rarely meeting the diagnostic criteria.
Rare autosomal dominant disorders of the PTEN pathway include Bannayan-Riley-Ruvalcaba syndrome (BRRS), Proteus and Proteus-like syndromes, and Cowden syndrome. The presentation within each of these clinical subtypes differs. Patients with BRRS have facial dysmorphic features that include a broad, flat forehead; thick eyebrows; full cheeks; long philtrum; and pointed jaw in addition to macrocephaly. Skin manifestations include papillomatous papules and café-au-lait spots; hyperpigmented macules are common on the glans of the penis.
In BRRS, the diagnosis is frequently made in infancy or childhood due to the significant intellectual disability and/or ASD seen in 25–55% of cases. Cowden syndrome is an adult-onset tumor predisposition syndrome, and Proteus syndrome is characterized by somatic overgrowth of the affected areas.
22q11.2 microdeletion syndrome has a broad array of phenotypic presentations that have many names including DiGeorge syndrome, velocardiofacial syndrome, Shprintzen syndrome, and conotruncal anomaly facial syndrome. This syndrome can be detected both early and later in life. Early presentations include congenital heart defects, cleft palate, hearing loss, immune dysfunction, and hypocalcemia.
The presence of cognitive impairment is common, but higher rates of ADHD and anxiety are also seen. Despite the presence of social-emotional reciprocity and nonverbal communication, this disorder is rarely characterized by repetitive behaviors or restricted interests, leading few patients to meet the ASD diagnosis.
The reciprocal duplication of the 22q11.2 deletion syndrome results in a disorder with more notable gross motor delays, although affected children struggle with abstract thinking and social abilities, which becomes more notable as school demands progress in the third or fourth grade. Receptive language is stronger than expressive; verbal memory is stronger than visuospatial memory.
These individuals can be described as shy or withdrawn, emotionally liable, hyperactive, and impulsive. They can move from task to task quickly but are less able to adapt to changes in routine or the environment. Social deficits are often seen including the lack of social-emotional reciprocity. They do not often have the repetitive behaviors and rigidity to fulfill the threshold for diagnosis of ASD. In one study 84% of cases had at least one psychiatric disorder, but only 18% met strict criteria for ASD.
This is an autosomal dominant disorder that results from pathogenic variants in either the TSC1 or TSC2 gene. The prevalence is estimated to be 1/6,000–9,000 people. Affected infants can be identified prenatally by the presence of cardiac rhabdomyomas as early as 22 weeks of gestation. Infants can present with infantile spasms, or during an evaluation for ASD. Most commonly it is identified postnatally with cardiac rhabdomyomas, with hypopigmented macules on the skin, or after the development of seizures. MRI will reveal subependymal nodules and cortical tubers.
IDD can be present, the severity of which is often associated with the age of seizure onset and their severity. Psychiatric comorbidity is significant, with 30–50% having ADHD, 30–60% with depression or anxiety disorders, and 25–50% with ASD.
Timothy syndrome is a neurodevelopmental disorder that is associated with long QT syndrome. It is caused by pathogenic variants in the CACNA1C gene, which encodes a calcium channel. The gene variant allows calcium to enter cells abnormally, which causes a prolonged QT interval that increases the risk of arrhythmias and sudden cardiac death. Physical examination can demonstrate low-set ears, lower nasal bridge, small upper jaw, and widely spaced teeth. Cutaneous syndactyly webbed fingers and toes are commonly found. Seizures, stroke, and blindness can also be found. Developmental delays, including ASD, are common.
Contiguous gene deletion of the 22q13 region that includes SHANK3 or pathogenic variants in SHANK3 results in Phelan-McDermid syndrome. SHANK3, which encodes for a scaffolding protein in the postsynaptic glutamatergic synapse, plays a critical role in synaptic function. While it accounts for a relatively small number of cases of ASD and IDD, the severity of the phenotype increases with the size of the affected region. While 20% of the cases are de novo, parents can carry a balanced chromosomal translocation, increasing the recurrence risk in additional offspring.
Most individuals have at least one visible dysmorphic feature including dolichocephaly, long eyelashes, pointed chin, prominent or dysplastic ears, bulbous nose, and full lips in addition to hypoplastic or dysplastic nails and large, fleshy hands. Hypotonia is often present and can present early in the form of feeding abnormalities and posture. Gait is almost uniformly affected and can range from nonambulatory to having issues with gait, toe walking, and broad-based ataxia. Coordination and motor planning issues are also seen in most cases. An increased risk of scoliosis is present.
Medical comorbidities are common. GI issues are most common and include gastroesophageal reflux disease (GERD), constipation, and diarrhea. Recurrent ear and upper respiratory infections can be linked to immune system abnormalities as well as allergies and asthma. There is an increased incidence of seizures. MRI data have included an increase in brain abnormalities including the corpus callosum, arachnoid cyst, ventriculomegaly, and dysmyelination. There can be significantly delayed or absent speech. Behavioral difficulties are often found but have not been well categorized.
There is an increased incidence of ASD in individuals with Phelan-McDermid syndrome, along with IDD.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here