Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Identification of chromosomal abnormalities often correlates to disease and phenotypic abnormalities and thus will aid in clinical diagnosis and treatment.
Identification of a cytogenetically abnormal clone in a cancer can provide information on diagnosis, prognosis, treatment, and disease progression.
Standard morphology and a specific banding pattern have been established for all human chromosomes. A variety of different technologies, including karyotype analysis, fluorescence in situ hybridization (FISH), microarray analysis, and deoxyribonucleic acid (DNA) sequencing, are used to uniquely identify chromosomes and determine if anomalies exist.
The two basic categories of cytogenetic abnormality are numeric and structural.
Many syndromes have been specifically linked to particular chromosomal anomalies (e.g., trisomy 21 in Down syndrome; 45,X in Turner syndrome; and deletion of 22q11.2 in velocardiofacial syndrome).
Cytogenetic abnormalities may be either de novo or inherited. Detection may occur at any stage of a person’s life from prenatal to adulthood.
FISH, a targeted assay that combines technologies from both cytogenetics and molecular genetics, can provide important diagnostic information about specific genes.
Cytogenetic analysis can be used in nearly every medical specialty and is an important element in clinical laboratory medicine.
Microarray analysis and DNA sequencing have identified new genetic syndromes. These technologies are key to optimizing genetic testing and treatment currently and in the future.
Genetics is broadly defined as the scientific study of heredity, but in clinical laboratory medicine interest is focused on two subspecialties of this field: human genetics, the study of heredity in humans, and medical genetics, the study of human genetic variation of medical significance. Medical genetics can be further subdivided into five groups, two primarily clinical fields (clinical genetics and genetic counseling) and three laboratory sciences (cytogenetics, molecular genetics, and biochemical genetics).
Groundbreaking discoveries in the field of medicine can be attributed to genetics. Research and the development of new techniques in molecular genetics and cytogenetics have identified new genes and mutations directly related to disease. Knowing the underlying cause of a disease aids in diagnosis, provides new options for therapy, and allows the possibility of curing some genetic diseases in the future. This chapter focuses on cytogenetics; discussion of molecular diagnostics is found in Chapter 72 .
As in all areas of medicine, genetics relies on the proper use of specific terms for communication. The following definitions provide a basic vocabulary for this field:
Gene: a sequence of nucleotides that represents a functional unit of inheritance; a region of DNA that codes for a product, either RNA or protein.
Chromosome: a highly ordered structure composed of DNA and proteins that carries the genetic information. In humans, there are 46 chromosomes ordered in pairs.
Autosome: all chromosomes other than the X and Y chromosomes, which are designated the sex chromosomes.
Homologous chromosomes or homologues: sister chromosomes, the members of a pair of chromosomes in which one is inherited from the mother and the other from the father.
Locus: the position of a gene on a chromosome.
Allele: an alternative form of a gene occupying the same locus. An allele may be the result of a mutation. There is a maximum of two alleles per diploid chromosome complement (one allele per chromosome), but multiple alleles may exist within a population.
Mutation: a permanent heritable change in the sequence of genomic DNA. This may manifest at both the molecular and cytogenetic levels. Not all mutations are negative events. Many are benign (e.g., blue eye color), and some have positive effects (e.g., sickle cell trait in countries with a significant risk of malaria). Individuals with a constitutional mutation (i.e., a mutation present in every cell of the body) may pass that mutation on to their progeny by germline transmission. In some cases, notably cancer, an acquired mutation may arise in a single somatic cell, which then divides mitotically, giving rise to a new clone of cells. The mutation will be limited to this clone and will not be transmitted to progeny of the individual. In rare instances of gonadal mosaicism , a de novo acquired mutation, may arise in the gonads, resulting in a mixed population of normal and mutant gametes. Progeny receiving the new mutation may display a phenotype not present in either parent.
Karyotype: the chromosome constitution of an individual.
Karyogram: a figure showing the paired chromosomes from a cell arrayed in a standard sequence.
Diploid: the presence of two copies of each unique chromosome per cell. In humans, the chromosomes occur in pairs and the diploid (2 N ) number is 46.
Haploid: one copy of each unique chromosome. In humans, the gametes are haploid ( N = 23).
Homozygous: both alleles at a locus are the same. (In the ABO system, an AA complement represents homozygosity.)
Heterozygous: the two alleles at a locus are different. (In the ABO system, an AO complement represents heterozygosity.)
Hemizygous: the presence of only one chromosome or chromosome segment rather than the usual two (e.g., males with a single X chromosome).
Genotype: the genetic constitution of an individual or organism (i.e., what alleles are present). (In the ABO system, AA, AO, BB, BO, AB, and OO are genotypes.)
Phenotype: the appearance of an individual that results from the interaction of environment and genotype. (In the ABO system, A, B, and O blood types represent the phenotypic expression of the alleles for a given individual.)
Dominant allele: an allele that is expressed when present in only a single dose (i.e., it “dominates” over the other allele present). (In the ABO system, A is dominant over O such that an AO genotype results in an A blood type phenotype. Similarly, the presence of pigment [T] is dominant to the absence of pigment [t] [i.e., albinism], such that Tt results in pigmentation.)
Recessive allele: in a diploid organism, an allele that is only expressed when homozygous. (In the ABO system, the O blood group is only seen with a OO genotype; O is recessive to A and B. Similarly, t is recessive to T, and an albino phenotype only occurs with a tt genotype.)
Codominant alleles: in a diploid organism, alleles that show no dominance or recessivity to each other, but when present together they are both fully expressed. (In the ABO system, A and B are codominant such that an AB genotype expresses both A and B antigens.)
Independent assortment: random assortment of chromosomes (paternal and maternal) in the gametes; there is a 50:50 chance of inheriting a given chromosome from one parent.
Linkage: the presence of two or more genes on the same chromosome that tend to be inherited together.
Crossing over: the physical exchange of genetic material between homologous chromosomes.
Recombination: the generation of new allelic combinations on chromosomes, usually by crossing over.
Mitosis: somatic cell division in which the DNA replicates and is evenly distributed to two equal daughter cells.
Meiosis: cell division in the gonads that produces the gametes. A single DNA replication is followed by two cell divisions that reduce the total DNA content of a cell from 2N to N. Recombination occurs to increase genetic diversity within a population.
Nondisjunction: failure of chromosomes or chromatids to separate to opposite poles in cell division. Usually results in one too many or one too few chromosomes in a cell.
Cytogenetics is defined as the science that combines the methods and findings of cytology and genetics, allowing the investigation of heredity at the cellular level. This involves careful evaluation of the chromosomes, structures composed of double-stranded DNA complexed with histone and nonhistone proteins. Chromosomes were first observed in tumor cells by Walther Fleming in 1882, only 16 years after Mendel established genetics as a new field of science. Thus cytogenetics is one of the oldest fields of genetics. Just after the turn of the century, the importance of the sex chromosomes was established, and in 1959, cytogenetic studies were first used in clinical laboratory studies. The ability to detect changes in chromosome structure and directly correlate them to disease and phenotypic anomalies in individuals proved a major advancement in clinical diagnosis. Over the years, the number and types of studies have grown, and many of the tests performed have become the gold standard for diagnosis. As more and more genes are being identified and linked to particular diseases, molecular testing, including direct mutation analysis, microarray, and sequencing, are becoming increasingly prevalent (see Chapter 69, Chapter 70, Chapter 72 ). However, cytogenetics continues to be the most cost-effective and straightforward test to detect chromosomal numerical and structural rearrangements. Consequently, clinical applications for cytogenetic analysis can be found in all age groups and genetic specialties, ranging from prenatal diagnosis to cancer evaluation ( ; ).
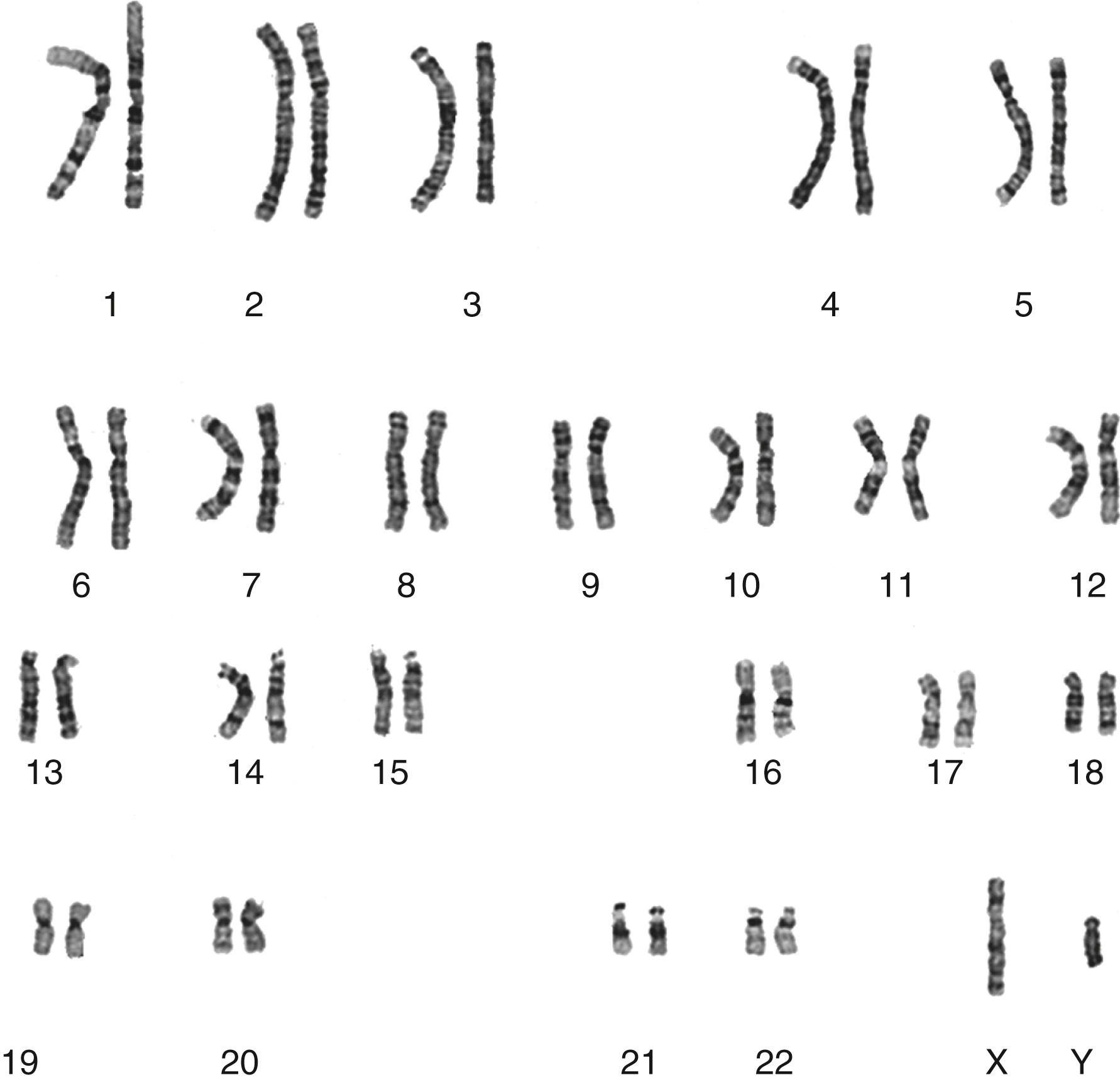
To recognize abnormalities, it is important to first understand what makes up a “normal” chromosome set. The human chromosome complement includes 46 chromosomes ordered in 23 pairs ( Fig. 71.1 ). One member of each pair is inherited from an individual’s mother and the other comes from the father. Twenty-two pairs are known as autosomes and appear as homologs of each other (i.e., they are indistinguishable from each other). The 23rd pair includes the sex chromosomes, which are homologous in a female, who has two X chromosomes, and are nonhomologous (structurally different) in a male, with one X and one Y chromosome (see Fig. 71.1 ). Genes are encoded in the DNA and are arrayed along the length of the chromosomes. During the cell cycle, the absolute lengths of the chromosomes vary, with the most condensed form reached in metaphase. This is the point when chromosomes can most easily be observed, so metaphase chromosomes are the basis for most cytogenetic studies. Complete evaluation of a set of chromosomes is known as karyotype analysis and an ordered image of the chromosomes from a cell is called a karyogram .
A single metaphase chromosome is composed of two DNA double helices associated with proteins. Each double helix is termed a chromatid, and the two chromatids are held together by an as yet unreplicated region of DNA known as the centromere, or primary constriction. In addition to its function in cell division, the centromere acts as a landmark and divides the chromosome into two distinct regions known as arms ( Fig. 71.2A ). The shorter of the two arms is designated the p arm, and the longer arm is known as the q arm . When the centromere is approximately equidistant from both ends, the chromosome is said to be metacentric, but if it is closer to one end than the other, the chromosome is submetacentric (see Fig. 71.2B ). Five pairs of acrocentric chromosomes have modified short arms with stalks containing only multiple copies of rRNA genes that are capped by a modified telomere termed a satellite (see Fig. 71.2A ).
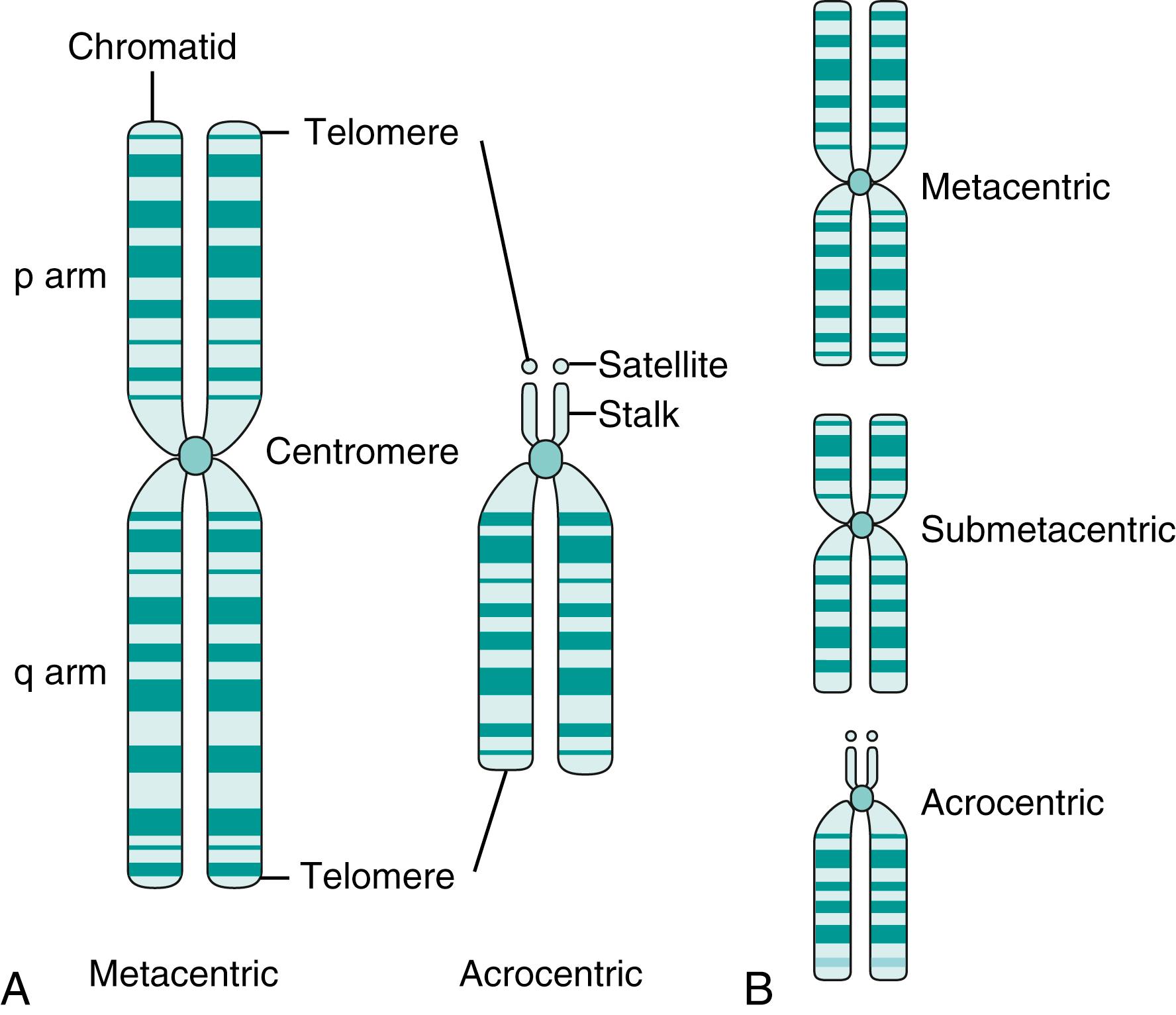
The end of a chromosome is the telomere . These regions are known to be composed of tandemly repeated DNA with the sequence (TTAGGG) n that functions to stabilize the chromosomes. The mechanics of DNA replication are such that not all of the telomere DNA can be replicated at each division, so there is a shortening of the telomeres over time. It has been postulated that total loss of the telomeres leads to an increase in chromosomal aberrations that may, in part, be responsible for carcinogenesis and the human aging process ( ; ; ; ).
To obtain metaphase cells for a chromosome analysis, cells from a patient must be cultured in vitro. The average human cell divides once every 24 hours, so only about 1% of the cell population is dividing at any given time. However, some cells, such as lymphocytes in a normal healthy individual, do not divide at all. Special culture techniques have therefore been developed to stimulate the cells to divide and increase the yield of metaphase cells.
Virtually any viable, nucleated cell sample can be used for cytogenetic analysis. However, certain types of cells are easier to obtain and culture and are therefore favored for chromosomal preparations. For routine cytogenetic studies of adults and children, heparinized peripheral blood is the preferred specimen. Obtaining a sample via standard phlebotomy is usually easy to arrange and relatively painless for the patient. In hematologic disorders, the best results are obtained from bone marrow samples because this is the origin of the disease. Fibroblast cultures from skin biopsies or skin punches provide an adequate source of metaphase cells. Tissues such as liver, kidney, lung, and muscle are not routinely used because of the invasive nature of acquiring such specimens; however, these tissues can be useful to evaluate a fetal loss or an autopsy. Products-of-conception samples may contain a mix of maternal and fetal tissue and so require extra care in establishing a culture ( ).
The most common specimen for prenatal analysis is amniotic fluid collected by amniocentesis. Under ultrasound guidance, the physician passes a needle through the mother’s abdomen and uterus into the amniotic sac. This is typically done between 16 and 18 weeks of gestation, at which time 20 to 30 mL of amniotic fluid (fetal urine) can be withdrawn without endangering the fetus. Cells present in the sample are derived from the fetus and provide a source of material for cytogenetic, molecular genetic, and biochemical assays. The fluid itself contains alpha fetoprotein (AFP), as well as other proteins and enzymes, and forms the substrate for other prenatal assays. The risk of fetal loss due to amniocentesis is approximately 0.2% to 0.3%.
Another prenatal procedure is chorionic villus sampling (CVS), which provides tissue from the developing placenta (chorionic villi) ( ; ). The transabdominal or transvaginal procedure is performed at 10 to 13 weeks of gestation and has a risk of fetal loss of approximately 1%. Because no amniotic fluid is collected, no AFP or related testing can be done, although standard cytogenetic, biochemical, and molecular analyses are possible.
Cordocentesis, or percutaneous umbilical blood sampling (PUBS), results in a fetal blood specimen that can be used for rapid karyotyping or molecular studies. This procedure is done at later gestational ages (≥20 weeks) and carries a higher risk of fetal loss (2–5%).
All clinical samples for cytogenetic analysis must be collected in a sterile manner. The presence of bacteria or fungi severely compromises the study because prokaryotic cells usually outcompete and overgrow any human cells that are present. To maximize the number of viable cells, specimens should be transported to the laboratory as soon as possible after collection. Blood, bone marrow, amniotic fluid, and chorionic villi should be maintained at room temperature, whereas solid tissue is transported on wet ice. The difference in temperature of transport is due to the native conditions of the sample. Blood, bone marrow, and amniotic fluid cells exist as individual cells in a fluid substrate, and the integrity of the sample will not be compromised as long as its temperature is maintained close to body temperature. However, collection of tissues such as skin requires cells to be excised from the body, leaving broken and dying cells at the periphery of the sample. The resultant release of lysosomal enzymes facilitates degradation of the dead cells, but the enzymes will also attack and destroy adjacent living cells. If the temperature is dropped to near 4°C, enzyme activity will be inhibited, and the viability of the sample is maintained.
Depending on the cell type, either a suspension (floating) or monolayer (fixed to a surface) culture technique may be employed. Blood and bone marrow cells are grown in suspension, so cells from the sample can be aliquoted directly into an appropriate culture medium. Bone marrow is typically cultured for 24 to 48 hours, whereas lymphocytes require 3 to 4 days in culture for maximum yield. Furthermore, because lymphocytes do not normally divide in culture, they must be induced using a mitogen, usually phytohemagglutinin. The resultant metaphase cells can then be collected by the use of a mitotic inhibitor, such as colcemid. Amniotic fluid cells, chorionic villi, and solid tissue are all grown as a monolayer in situ. Tissue and chorionic villi are first disaggregated using a mild collagenase treatment, and the individual cells are then seeded onto glass coverslips and covered with culture medium or placed into culture flasks. In an amniotic fluid sample, the cells must first be separated from the fluid by centrifugation before being plated in dishes or flasks and allowed to form in situ colonies. Typical culture times are 5 to 7 days for chorionic villi and amniocytes and up to 2 weeks for solid tissue culture.
Once maximum growth has been achieved, all cell types are harvested using similar techniques. The cells are swelled hypotonically (to the point that the cell membrane is stretched but not broken) and are then fixed, typically using a methanol:acetic acid fixative. Gentle blowing on the coverslip containing the fixed cells from in situ cultures results in the rupture of the cell membrane and a spreading of the metaphase chromosomes. For suspension cultures, the fixed cells must be dropped onto a clean microscope slide, which mechanically breaks the membranes and leaves the chromosomes separated slightly from each other but in a discrete region occupied by a single cell ( Fig. 71.3 ). After air drying, the slides are suitable for staining. In some cases, artificial aging of the cells at approximately 65°C for 30 to 60 minutes improves the quality of the staining. Although most laboratories still perform these steps manually, an automated robotic harvester has been developed that can process large numbers of cultures quite efficiently.

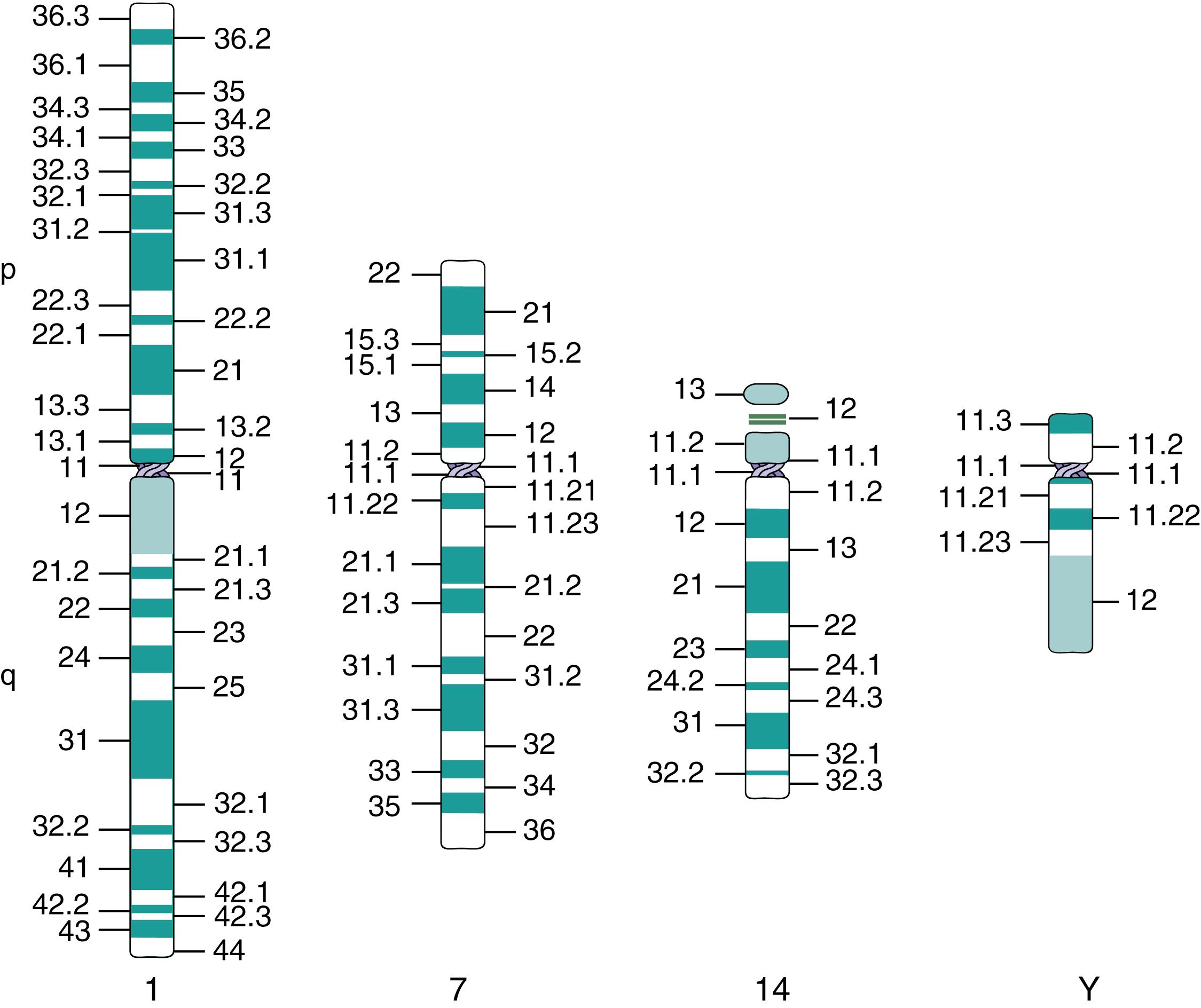
Chromosomes are routinely stained using Giemsa or Wright stain, positively charged dyes that bind to the negatively charged DNA molecule. Mild trypsinization of the chromosomes before staining apparently weakens the DNA-protein interactions, yielding a defined pattern of alternating light and dark regions after the stain is applied. This is called the banding pattern (G-banding for Giemsa banding) ( ; ; ). Each pair of chromosomes has a unique band pattern that is schematically represented as an ideogram ( ) ( Fig. 71.4 ), and this is used to identify each chromosome and the chromosomal subregions.
Although rarely used today, additional information about chromosome structure can be obtained with other special staining technologies. The most common special stains include Q-banding, C-banding, and R-banding. Q-banding , or quinacrine fluorescence staining, was originally used in routine chromosome analysis ( ). However, because the fluorescence is transient, it has been replaced by G-banding, which allows a permanently stained preparation ( ). The current major application of Q-banding is for rapid identification of the Y chromosome. The distal end of the long arm of the Y chromosome is composed of heterochromatin and is the most brightly fluorescent region in a human metaphase ( Fig. 71.5A ).
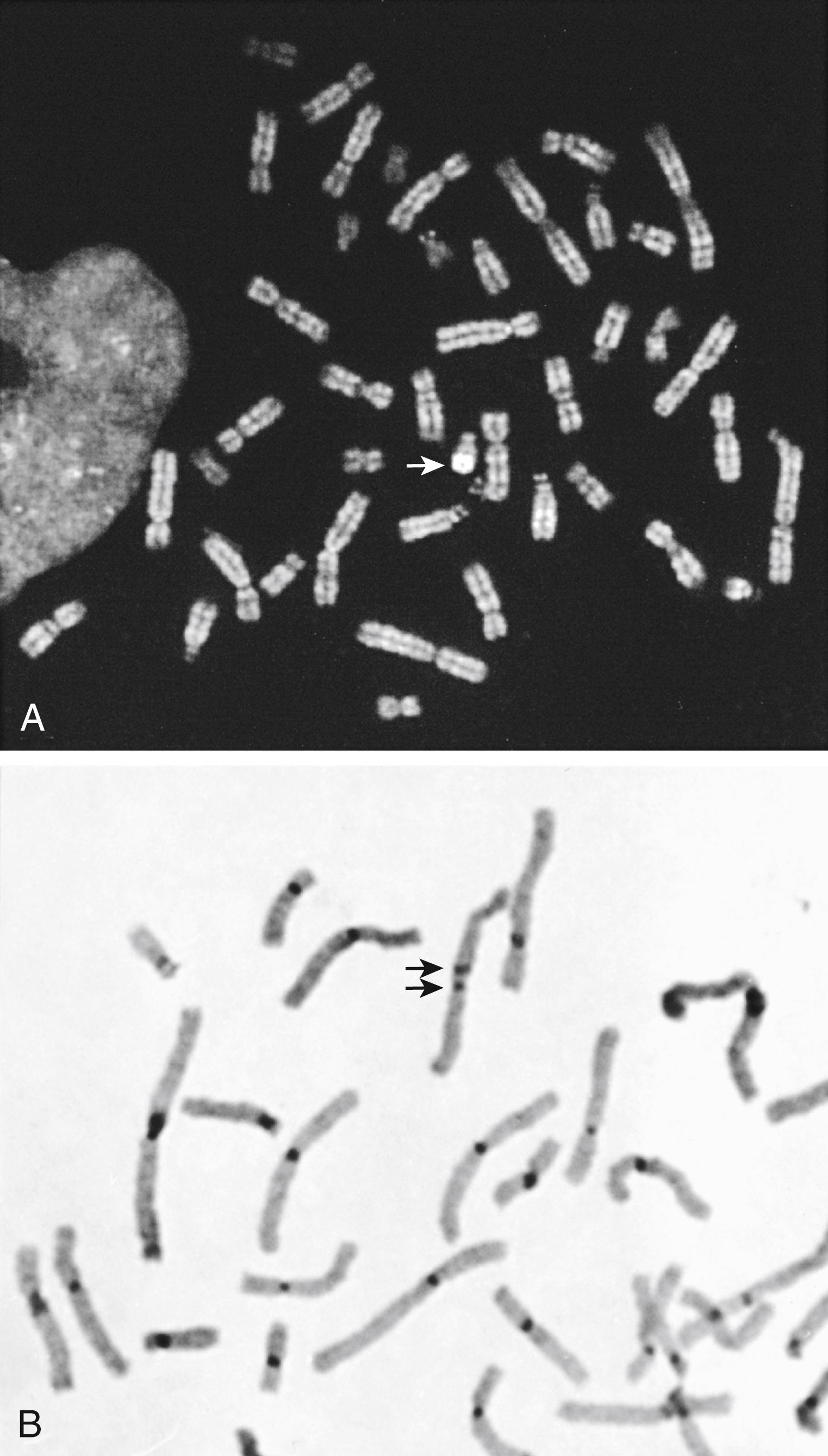
C-banding , constitutive or centromere banding, may be used to evaluate constitutive heterochromatin or to determine whether a chromosome has two centromeres (dicentric). Normally, a centromere appears as a single dark spot on an overall pale-staining chromosome ( ). In the case of a dicentric chromosome, the presence of two dark regions clearly identifies the two centromeres (see Fig. 71.5B ). R-banding , or reverse banding, results in chromosomes with the same banding pattern as that seen in G-banding, but the light and dark bands are reversed, hence the name. Because the telomeres of the chromosomes tend to be small, light-staining bands, a deletion may not be easily detected using standard G-banding. In R-banding, however, the telomeres should appear as dark bands, and their absence as the result of a deletion is more obvious.
To perform a cytogenetic (karyotype) analysis, it is essential to be able to rapidly and accurately identify each chromosome and determine when chromosome abnormalities are present. The first step is to count the number of chromosomes present in the cell being evaluated. The number of active centromeres defines the total number of chromosomes and will total 46 in a normal human diploid cell. Too many or too few chromosomes indicate a potential numerical abnormality. In vitro culture can result in culture artifacts, so no single cell is used to define an individual’s chromosome complement. A typical clinical study requires analysis of 15 to 20 cells. Single cells with abnormalities are considered artifacts. Three or more cells with the same chromosome loss or two or more cells with the same additional chromosome or structural rearrangement define a true abnormality. In cases of mosaicism or for other special situations, 10 to 30 additional cells may be evaluated.
Individual chromosomes are identified based on the overall size of each chromosome, the position of its centromere, and the banding pattern. Any variation in chromosome structure should be detected at this time. In most circumstances, routine G-banding of metaphase chromosomes is sufficient for clinical diagnostic purposes. However, some disorders are associated with very small deletions of chromosomal material that may not be resolved at this level, so high-resolution analysis is used. Special culture conditions are employed, and the cells are harvested at prometaphase, a slightly earlier stage of cell division. At this point in the cell cycle, the chromosomes are less condensed and physically longer, making the presence of small abnormalities easier to detect. Please note that even at high resolution, the ability to detect very small chromosome abnormalities is limited. A duplication or deletion of about 3 MB is the smallest that can be seen using conventional karyotype analysis.
The analysis is performed by a technologist viewing the cells at the microscope, and once the study is complete, a determination is made as to whether the chromosome complement is normal or abnormal. For documentation, representative metaphase cells are captured, and karyograms (illustrations of the chromosomes of a given cell) (see Fig. 71.1 ) are prepared. The homologous pairs are arrayed from large to small, with special placement for the pair of sex chromosomes. By convention, the shorter arm, the p arm, is oriented up, and the longer q arm is oriented down.
To capture the images, cytogenetics laboratories now use computer-assisted imaging. A CCD (charge-coupled device) video camera is used in combination with specially designed software to capture an image of a metaphase cell, which is digitized and displayed on the computer monitor. The image can be modified by lightening, darkening, or changing the contrast, and a variety of manipulations of the chromosomes are possible, including straightening and importing chromosomes from other fields. A karyogram can then be generated using a pattern-recognition subroutine that will automatically identify the chromosomes and place them in their proper places on a karyogram form. Depending on the software and the quality of the image, the accuracy of this process can vary from 10% to 85%. After the computer has taken its “best guess,” a trained cytogenetic technologist must make the appropriate corrections, and the final version of the karyogram can be printed out by a high-resolution printer and then archived. A routine karyogram from capture to printing should take a trained technologist on average 15 to 20 minutes.
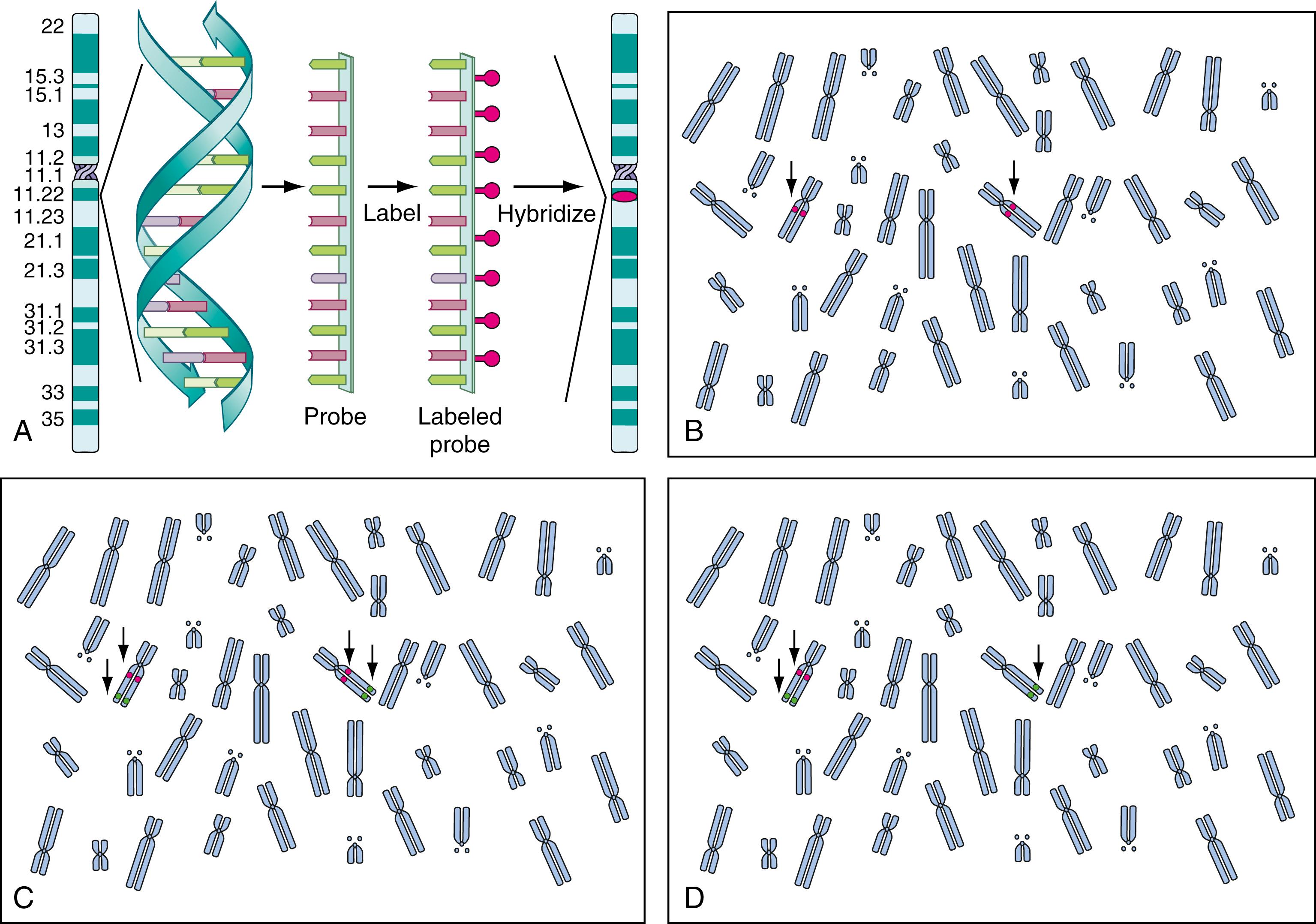
FISH, a combination of molecular and cytogenetic technologies, has expanded the ability to investigate chromosome anomalies. This technique requires a molecular probe (i.e., a fragment of DNA) that is labeled with a fluorescent dye. To create a probe, a target DNA of interest is chosen, the relevant DNA is isolated, and a single stranded copy of that DNA is labeled with a fluorescent dye. This probe can be hybridized to denatured metaphase or interphase cells and should bind to the homologous region of the chromosome from whence it was derived. The presence or absence of the signal can be seen using a fluorescence microscope ( ).
In clinical applications of FISH, the most common goal is to determine whether a gene, a specific mutation, or a particular chromosomal rearrangement is present, so the molecular probes used must be well characterized and specific to the locus in question. There are three basic types of probes. Chromosome painting probes are actually a cocktail of many unique DNA fragments from along the entire length of a chromosome, so that, following hybridization, the entire chromosome fluoresces ( Fig. 71.6A ). Repeat sequence probes are usually used in chromosome enumeration (i.e., to detect the gain or loss of specific chromosomes) ( ). Chromosome-specific pericentromeric and subtelomeric probes are typically used for this purpose (see Figs. 71.6, C and D ). A unique sequence probe is usually isolated from cloned DNA of a disease-causing gene or a fragment of DNA of known location associated with a particular gene. This type of probe is used to identify the presence or absence of a gene, gene region, or chromosomal rearrangement of interest ( ; ). Subtelomere probes are a subset of this category, and chromosome-specific subtelomere probes have been used to characterize cryptic subtelomeric deletions or rearrangements that have been associated with unexplained mental retardation (see Fig. 71.6B ) ( ; ; ; ; ). Although accurate and useful, the subtelomere FISH assay has now been largely replaced by chromosome microarray studies.
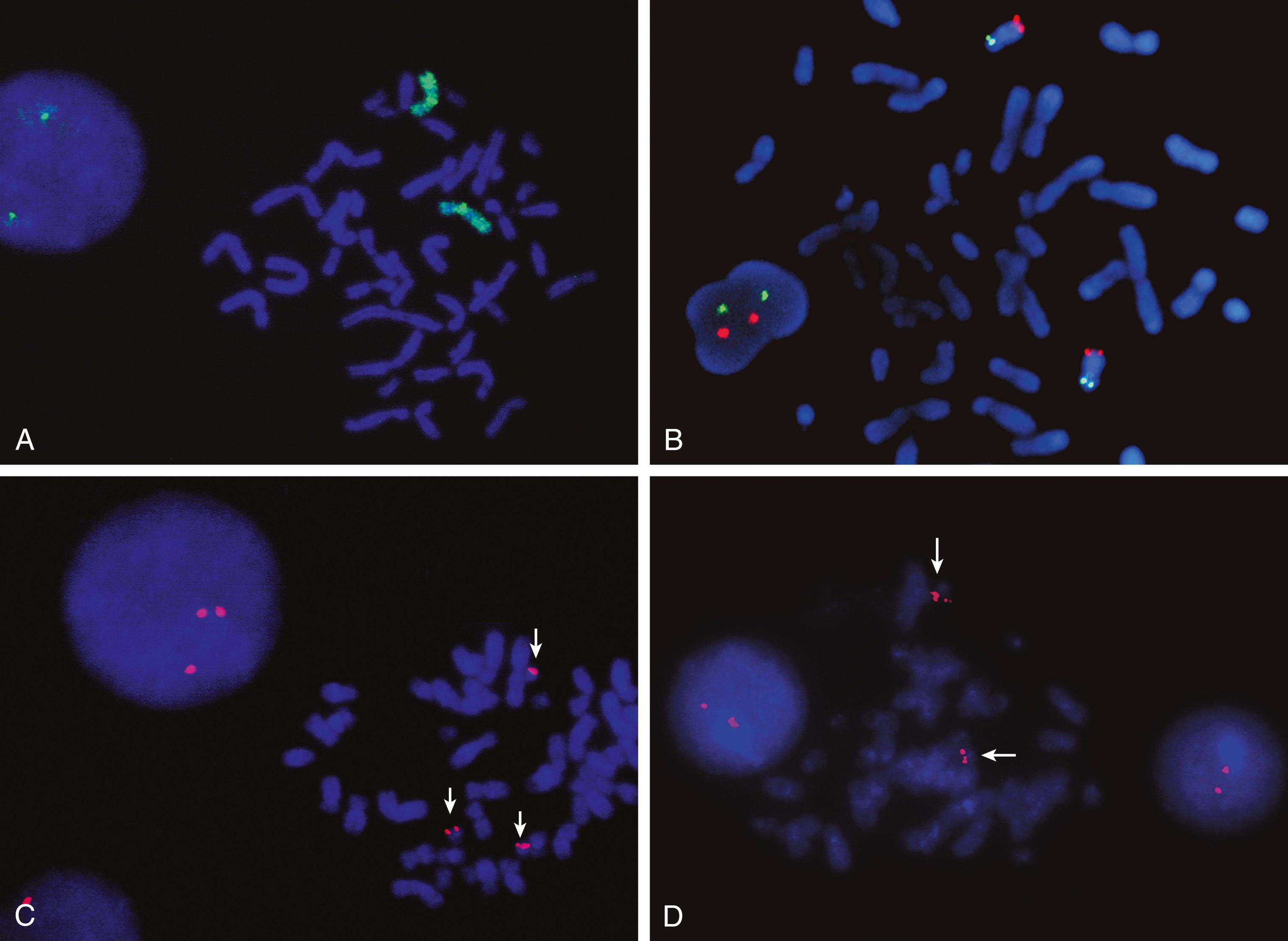
FISH can be performed on either metaphase or interphase cells. For metaphase FISH, cells are cultured and harvested as for a routine karyotype analysis, but no culture is required for interphase FISH. Fixed cells are collected, and slides are made as described for karyotyping. The DNA on the slides is then denatured, and a fluorescently labeled single-stranded molecular probe is allowed to hybridize to the chromosomal DNA using an annealing temperature that favors hybridization of homologous regions of DNA ( Fig. 71.7A ) . After an appropriate period of hybridization, excess probe is washed away and the nonhybridized DNA is counterstained with another fluorochrome to allow visualization of the entire chromosome complement. A fluorescent microscope with light from a 100-watt mercury bulb in combination with appropriate sets of exciter filters allows evaluation of the cells. Due to the optical limitations of the glass filters, a maximum of three colors can be visualized on a typical fluorescent microscope. For documentation, images are captured using a computer-assisted system.
One of the most critical elements in FISH is the selection of a specific probe(s) that will help to answer a clinical question. For example, one of the most useful clinical applications of FISH is the detection of microdeletions too small to be seen using classical cytogenetics (see Fig. 71.7B-D ). The gene must be known, and the probe used must be homologous to the critical region of the gene that is usually deleted. Hybridization with the probe should result in a fluorescent signal only at the target locus. If a signal is present, DNA complementary to the probe is present, so there is no deletion (see Fig. 71.7B ). However, the absence of signal indicates that a deletion exists (i.e., there is no DNA sequence present on the chromosome that is complementary to the probe, so no hybridization can occur) (see Fig. 71.7D ). A control probe to a different region of the chromosome being tested is usually used as a hybridization control (see Fig. 71.7C ). Therefore, when evaluating a disease-related probe, an unaffected individual should have two signals per cell for each autosomal gene: one signal for each chromosome of the pair ( Fig. 71.8A ). An affected individual with a deletion should have a single signal per cell, showing one normal and one deleted chromosome (see Fig. 71.8B ). For both affected and unaffected individuals, the control probe should show two signals per cell in all cells that are scored. In a metaphase cell, the chromosomes may be short and condensed, with each chromatid individually visible, resulting in a discrete FISH signal on each chromatid. In these cells, there may appear to be two signals per chromosome, for a total of four signals, but in actual scoring only complete chromosomes are considered so a signal on one or both chromatids would be counted as just one positive signal.
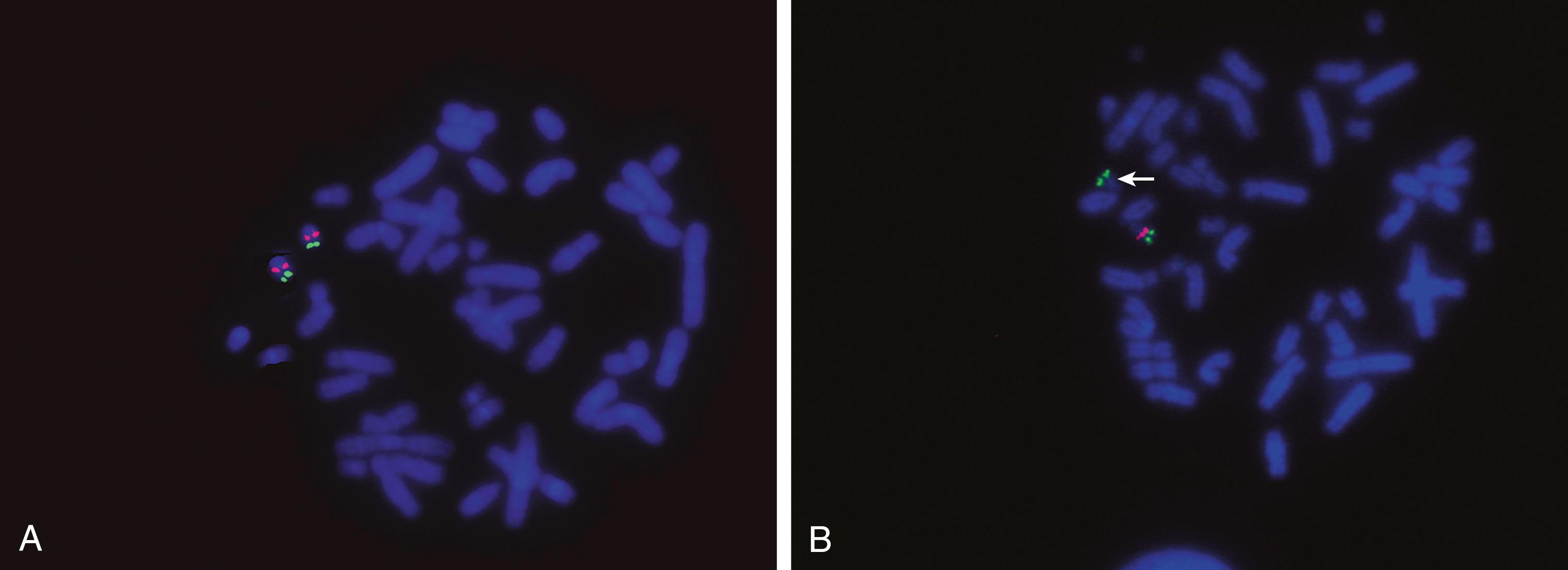
One of the problems with this detection system is that the lack of signal is the positive indication of a deletion. Technical failure of hybridization may also result in absence of a signal. To eliminate this as a source of error, a minimum of 20 cells must be evaluated, and all cells must agree in signal count. If mosaicism is suspected, additional cells may be surveyed. In addition, unique sequence probes are currently used in combination with a second probe—a control probe—that is localized to a different locus on the same arm of the target chromosome (see Fig. 71.7C ). The two probes are usually labeled with fluorochromes of different colors. In all cells evaluated, two clear control signals must be seen, and then the corresponding signals for the disease locus can be recorded. This provides a hybridization control as well as a marker for the chromosome of interest (see Fig. 71.7D ). If a dual-color system is used, results may be obtained in interphase as well as metaphase cells.
Chromosome painting probes are most useful in identifying complex rearrangements or marker chromosomes. If an individual has an abnormal chromosome with extra material of unknown origin, it may be possible to use chromosome painting to identify the source of the extra DNA. This may help in either diagnosis or prognosis of the individual. For example, in the case of a patient with only one identifiable X chromosome and a small, unidentified marker chromosome, it will be important to determine if the origin of the marker is an X or a Y chromosome. This can be accomplished using chromosome paints for the X and Y chromosomes labeled with different fluorochromes.
FISH is a targeted assay. The probes will only provide information about the locus from which they are derived. Thus when interpreting results, it is critical to know which probe was used. When using metaphase cells, the necessary data can usually be obtained from viewing about 20 cells. A larger sample of interphase cells (200–500) can yield statistically significant data about the frequencies of disease clones.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here