Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The laboratory mouse has long been used as a biomedical tool. Over the past 50 years, the mouse has transformed into the standard model to study and compare their pathophysiology and genetics to a wide variety of human skin diseases and many others. These mouse models were found for many decades as naturally occurring (spontaneous), radiation, or chemical mutagen-induced genetic-based diseases. With the advent of transgenesis (moving genes from one species to another or overexpressing a gene) during the late 1970s, to gene targeted (so-called knockout) technology in the late 1980s, then inducible models, where genes could be selectively turned on or off with or without markers in the late 1990s and early 2000s, to the current state-of-the-art endonuclease modified models, that offer the possibilities of molecular correction of the germ line for genetic-based diseases, the laboratory mouse has become a singular utilitarian biological tool and powerful dermatology surrogate. Information on methods to create these mice is available elsewhere but is summarized below. Continuing breakthroughs utilizing these mouse models have provided enlightenment on the pathogenesis of countless diseases of all organ systems including the skin and adnexa. However, mice are not the only model organism available. The zebrafish has also provided a great deal of basic information on development and genetic-based diseases of the skin. While anatomically different from the mouse, it nevertheless provides a number of advantages including serving as a second commonly used vertebrate species for genetic-based studies speed of production. This chapter provides an overview of a few of the constantly expanding types of mouse and zebrafish models for human skin diseases that exist and the context of how they can be used to dissect complex molecular pathways. While genetically engineered mice (GEMs) have opened up research to almost anyone to create and phenotype mice with targeted mutations, allowing partially or complete gene inactivation, these technologies have also caused many to question if mice can accurately model human diseases, as these types of mutations, total loss of function of a gene and the protein it codes for, are rarely found in humans. True null (total deficiency of protein production) mutations do occur naturally in humans, as in mice, but these mutations often result in embryonic lethality and fetal resorption, so no offspring are actually born to be analyzed. This has been one of the findings in the Knockout Mouse Project (KOMP), where over 20% of the null mutations result in embryonic lethality. The KOMP mice are inbred while most humans are not, hence the offspring are more likely to show a lethal phenotype. Spontaneous mutations in mice often very accurately recapitulate human diseases and, in a number of cases, have provided the knowledge to correctly define new human diseases. For example, the rhinoceros (now called the rhino) mouse (a severe allele of hairless) was reported in 1859, the human homolog in 1954, and the correlation of the two in 1989. The lanceolate hair mutant mouse ( Lah ), first reported in 1996, led to the discovery of desmoglein 4 ( Dsg4 ) in the mouse and eventually to the novel human disease, localized autosomal recessive hypotrichosis (LAH), the name of which was modified to fit the mouse locus symbol 6 years later. With the advent of the CRISPR/Cas9, investigators can now make precise germline mutations in mice (and other species). When matched with mutations in patients with specific diseases, a similar if not exact duplication of the human mutation (rare variant) can now be created in mice. This duplication can be simply a point mutation in a homologous gene and location to total replacement of the mouse gene or genetic region with a human gene carrying the mutation of interest, i.e., humanization of the mouse genome. As these technologies continue to evolve, increasingly more accurate mouse models recapitulating specific subsets of human diseases will provide increasingly improved outcomes.
The second critical aspect of developing highly accurate mouse models is carefully and comprehensively defining the phenotype (i.e., accurate diagnosis) with comparison to similar human diseases to find the best match. This goes beyond simply evaluating the organ of interest in the mouse model and requires in-depth studies on all other organs in the model. Most mice used in research are inbred, meaning that within a strain all mice are essentially genetically and phenotypically identical except for their sex. This limited genetic diversity within a strain results in a highly reproducible phenotype. Such phenotypes may not represent all aspects of a specific human disease because humans are not, in general, inbred. As such, the wide genetic diversity between most humans impacts the phenotypes of many diseases creating the variability commonly seen among human patients; however, the variety and genetic diversity available in many inbred strains can allow researchers to compensate for the lack of genetic diversity within a single strain. By taking advantage of this knowledge, mouse models are increasingly becoming powerful tools to dissect the modifier genes responsible for subtypes of diseases commonly seen by dermatologists.
The one gene, one enzyme hypothesis put forth in the early 1940s by George Beadle was influenced by the results of experiments looking at the inheritance of coat color differences in mice. Over the next few decades, this same theory was proposed for genetic disease in general, whereby a mutation in one gene affects one disease state. Currently, we know that phenotypes, regardless of type, are not usually the result of mutations in single genes, but rather of the interaction of molecular pathways involving multiple gene products. Genes do not act in isolation, but evolved over time to both function and segregate together under natural selection to provide the organism with a related interacting set of alleles. This is illustrated by the existence of genocopies, i.e., an individual whose phenotype or disease diagnosis mimics another determined by a distinct gene or assortment of genes ( http://medical-dictionary.thefreedictionary.com/genocopy ). By contrast, a phenocopy is an environmentally induced phenotype (i.e., disease) mimicking one usually produced by a specific genotype ( http://medical-dictionary.thefreedictionary.com/phenocopy ). Laboratory mice are maintained in highly environmentally controlled rooms which can reduce environmental effects (phenocopies). The question is whether a group of interacting mutant alleles causing similar phenotypes (genocopies) is advantageous or deleterious and how to identify the genes and define their function. The mouse is the perfect tool to answer these questions regarding the linkage of genes relating to specific phenotypes. Observing mice within an inbred line allows us to study environmental inputs against a stable genetic background (phenocopies), whereas observing mice across multiple inbred lines allows the discovery of both unique and new combinations of alleles affecting a particular phenotype and molecular pathway (genocopies). Each inbred strain varies, with respect to genetics, and these variations will affect interpretation of the phenotype and more importantly, how the inbred mouse compares to a specific human disease. This is known as the ‘Cinderella effect’, where knowing these differences can help fit the mouse model (like a shoe) on specific backgrounds to a specific form of a human disease.
At the gross, microscopic, and molecular levels, the skin, hair, and nails of most mammals, including humans and mice, are fundamentally the same. Because of this basic anatomical phylogenetic conservation between mammals, many diseases are also, not surprisingly, very similar. However, when one first compares a mouse with a human more closely, the two species can show clear differences. Similarities and differences between mouse and human skin are summarized in Table 36.1 and described in greater detail elsewhere.
| Criteria | Human | Mouse |
|---|---|---|
| Epidermis of body (thin) skin | Thick (50–100 µm) | Thin (10–15 µm) |
| Epidermis of thick skin (plantar/palmer surfaces) | Thick (300–400 µm) Palms of hands, soles of feet |
Tail (70–80 µm), footpad (150–400 µm), muzzle (20–30 µm), eyelid (50–60 µm) |
| Hair types: body | Vellus Terminal |
Guard Auschene Zigzag Awl |
| Specialized hair types | Cilia (eyelashes) Eyebrows Pubic Facial (beard, mustache) Axillary Ear hair |
Cilia (eyelashes) Vibrissae (specialized somatosensory organ with blood-filled sinus around the muzzle, eyes, and feet) Perianal Tail hair Ear hair (inner and outer pinnae are different) |
| Emergence of hair | 19–21 weeks gestation | 5 days postpartum |
| Hair cycle | Mosaic pattern | Wave pattern of whole body as neonates Wave pattern within zones in adults |
| Tail and tail hairs | No (no tail) | Yes |
| Sebaceous glands | Yes | Yes |
| Modified sebaceous glands | Meibomian glands Ceruminous glands |
Meibomian glands Zymbal gland Preputial gland Clitoral gland (in females) Perianal gland (in males) |
| Eccrine glands | Found on entire body including soles and palms | Limited to footpads |
| Apocrine sweat glands | Axilla, genitoanal region, ceruminous glands, mammary glands | Mammary glands present in most skin including limbs Mice do not have ceruminous glands or apocrine glands in the dermis of the body skin |
| Nails | All distal digits | All distal digits |
The skin of the body (or torso) of the mouse, usually referred to as the truncal skin, is very similar to human skin. However, while the epidermis is relatively thick for the first 2 weeks of life, it thins out and remains very thin throughout adulthood. The same layers can be found in the epidermis as in other mammals, the stratum corneum, granulosum, spinosum, and basale from the most superficial layers to the basement membrane. There are no rete ridges in mouse skin. The dermis consists of dense regular collagenous connective tissue. Below the dermis is a layer of white fat (hypodermal fat layer) that varies in thickness with the hair cycle. A layer of skeletal muscle, the panniculous carnosus, is located below the hypodermal fat. The mouse truncal skin has four types of hair follicles (guard, auschene, awl, and zigzag) producing different types of hair shafts ( Fig. 36.1 ). Whole slide scans of mounted plucked normal mouse truncal hairs can be viewed online ( http://eulep.pdn.cam.ac.uk/zoomify/index.php?image=288&mutant=Normal%20Plucked%20Hairs ). Guard hairs are very large with long hair follicles that produce a large hair shaft. These large hair shafts extend above all others and also have a sensory function. Auschene and awl hairs are intermediate forms between guard hairs and zigzag, the last of which are most numerous and are the underhairs that produce lift and thermal insulation. The structure of the hair shaft types is different. The medulla is well developed in mice and consists of either a single layer of cells in a septate pattern or two or more lateral rows of cells called a septulate pattern (see Fig. 36.1 ).
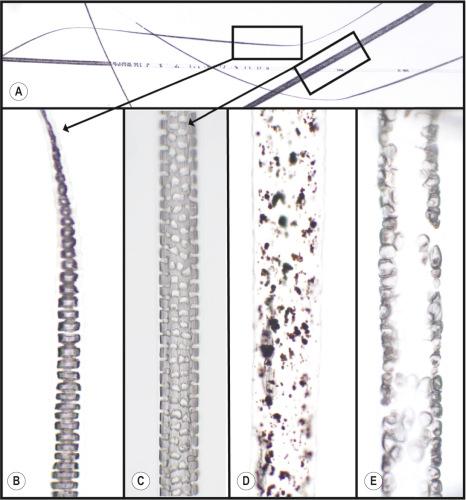
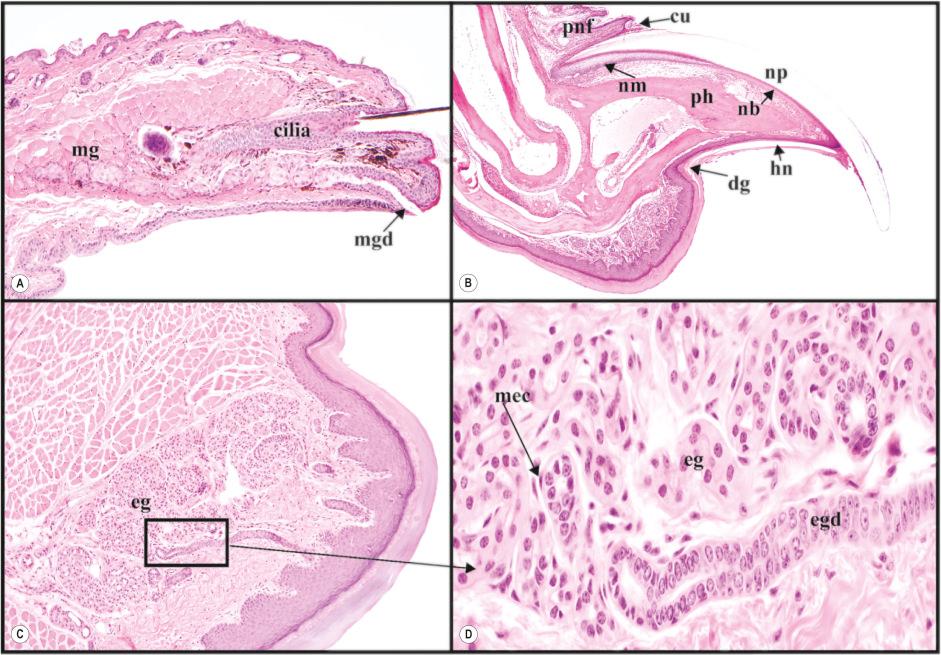
In addition to the four truncal hair types, mice have a variety of specialized hairs, some with human counterparts and others that are found in other mammals but not in humans. The most notable of these unique mouse hairs are called vibrissae, which are located around the muzzle, above the eyes, and just above the wrist and ankle regions on the legs. Vibrissae are most prominent along the muzzle where they form prominent rows with very long hair shafts, i.e., whiskers. These are large somatosensory organs surrounded by blood-filled sinuses ( Fig. 36.2 ).
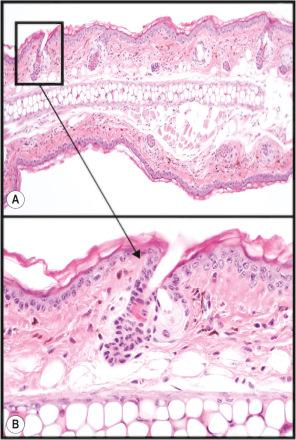
Eyelids have a row of large follicles that produce a thick but short hair shaft, the eyelash (cilia) ( Fig. 36.3A ), which serves as a sensory organ to protect the eye, reduces deposition of airborne particles, and reduces evaporation of the tear film. A row of large modified sebaceous glands (Meibomian glands) have a single duct that empties independent of this follicle at the mucocutaneous junction. This gland produces lipids to cover the cornea and which helps to reduce evaporation.
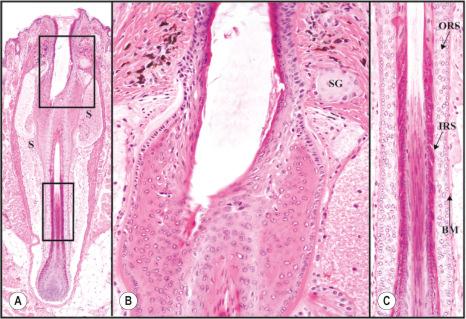
The mouse nail unit is fundamentally similar to that found in humans except obviously in size and gross appearance. Distinguishing features between the species are the shape of the nail and the presence of an extended hyponychium in the mouse ( Fig. 36.3B ). Human nails form a plate while the mouse nail curves around the distal digit forming an almost tube-like structure. Both species have a proximal nail fold, cuticle, nail matrix containing stem cells, nail bed, nail plate, and hyponychium. Gene expression patterns of most keratins are similar. These findings indicate that the mouse nail unit shares major characteristics with the human and overall represents a very similar structure, useful for the investigation of nail diseases and nail biology.
Like human hands and feet, there is no hair on the footpads in the mouse, the equivalent of the human acral sites of palms and soles ( Fig. 36.3C–D ). The footpad is thicker than the body (truncal) skin and the footpad contains eccrine glands, just like humans. However, mice do not have fingerprints based on scanning electron microscopic studies.
The tail is an anatomic structure not usually found on humans. The mouse tail epidermis is thick, relative to the truncal skin, and has a regular arrangement of large follicles that produce wide, short hairs ( Fig. 36.4 ). While the tail appears to be without hair in normal adults, careful examination at the gross level reveals there are fine, short hairs present. The follicles grow in triplets, and where the hair shafts emerge the epidermis is thrown into folds producing a scale-like appearance to the skin.
Ear skin in the mouse is also unique and different from human ear skin. The epidermis is thin, as it is on the trunk. However, there are uniform small short hairs and hair follicles on either side of the pinna that are different from truncal hair ( Fig. 36.5 ). Ear hair shafts are very short, have a short anagen stage, and have very prolonged telogen stage. As such, ear hairs are rarely seen in anagen in histologic sections.
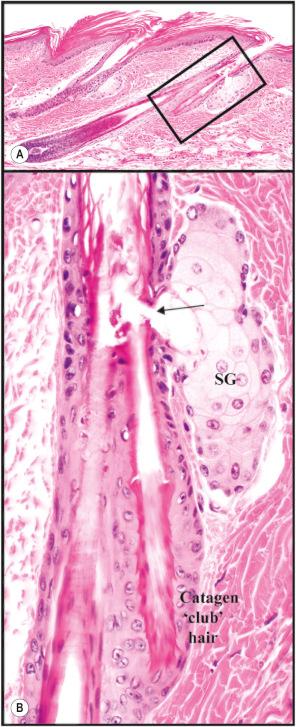
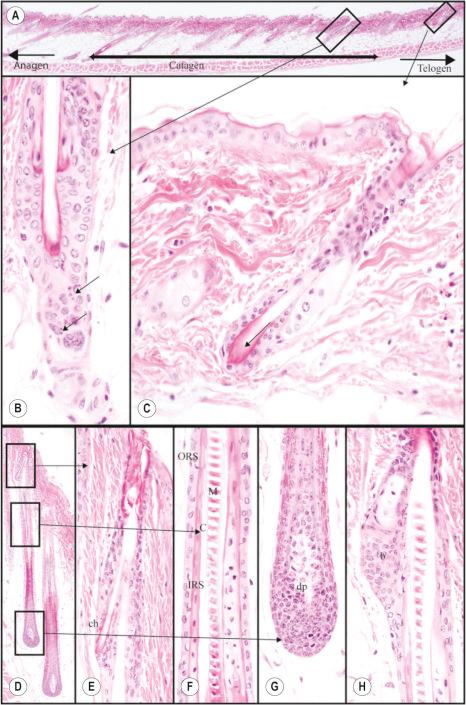
Hair follicles cycle in mice as they do in humans ( Fig. 36.6 ); however, a major difference is that in mice the hair cycles in an anterior to posterior (A–P or head to tail) direction throughout life, in what has been described as a wave pattern as opposed to a mosaic pattern which occurs in humans. In adult mice, this wave becomes more regional but continues in an A–P direction. This characteristic of mouse skin provides a research advantage in that one can see most, if not all, of the stages of the hair cycle in one histologic section if carefully selected. Another difference is that anagen is a relatively short part of the cycle with adult mouse follicles remaining in telogen for long periods.
Pigmentation in mice is usually not found in the interfollicular epidermis in most inbred strains of mice, but rather it is located primarily within the anagen hair follicles. This enables one to follow the hair cycle in an adult pigmented mouse by simply shaving them and watching the skin color change from pink (telogen) to light gray (early anagen) to dark gray (late anagen).
The variation in skin and hair follicles at these different anatomic sites are often considered minor variations in the same anatomical structures; however, transcriptome studies of skin biopsied from different anatomic sites revealed that in mice and humans these sites are fundamentally different organs. This observation is validated by the fact that several single gene mutations in mice result in skin disease at very specific anatomic sites. For example, the chronic proliferative dermatitis mutant mouse ( Sharpin cpdm ) results in a psoriasiform dermatitis of the dorsal and ventral trunk, yet the ears, tail, and distal extremities are spared. Modified sebaceous glands, such as the preputial or clitoral glands (structures not found in humans), may be completely missing in some mutant mice, such as Hoxd13 spdh mutant mice, while all other sebaceous and modified sebaceous glands are unaffected.
Methods for analyzing the skin are largely applicable to all species of mammals and are well described specifically for the mouse elsewhere. One common question is what is the best fixative for mouse tissues for histopathology? That which you are most familiar with is the best answer, as any artifacts will be similar to what you are used to. Many use neutral buffered 10% formalin, which is quite effective for mouse tissue as it is for human tissues. However, a less commonly used fixative, Fekete acid-alcohol-formalin, used as an overnight fixative followed by storage in 70% ethanol, provides highly reproducible results with the added benefit of better epitope retention for immunohistochemistry than most commonly used fixatives, including neutral buffered formalin. Specific assays of value for mouse skin focus on the hair cycle. As mentioned above, the hair shaft pigmentation reflects the anagen stage of the hair cycle. This can be used as a tool to study the effects on the hair cycle of drugs or a genetic mutation by shaving the mice and watching the pink skin (telogen) change to pigmented skin (anagen). The hair cycle can also be induced by wax stripping, using the same tools as humans used for cosmetic depilation.
As detailed in Table 36.1 , the mouse shares many characteristics with humans, making them powerful models for many human disorders. Furthermore, their use has been greatly extended in recent years with the development of genetic modification technologies allowing modification of the murine genome to be almost routine. However, it is with the advent of human genome-wide association studies (GWAS) and modern sequencing methodologies allowing the identification of associated genetic variants correlating with human disease that has really empowered the mouse model in genetic research. When these new systems are combined, i.e., human GWAS putative causal links with the ability to make similar genetic lesions in mice, it is now possible to test the genetic causal correlation and make available mouse models which presents similar disease phenotypes. With regard to approaches to make these precise genetic modifications, the key breakthrough leading to rapid, economic modification of mice (and other species including zebrafish) was the development of the targeting nucleases ZFN (zinc finger nuclease) and TALEN (transcription activator-like effector nuclease), with a major further reduction in cost and complexity with the more recent harnessing of the CRISPR/Cas9 system.
At the time of writing, the method of choice in creating genetically modified mice is based on using CRISPR/Cas9. The approach is executed directly in zygotes derived from any desired mouse background. The method uses a guide RNA (gRNA) that carries 17–20 base pairs with sequence homology to the desired genomic target. Upon introduction of the gRNA plus Cas9, generally by microinjection directly into zygotes, a complex forms leading to a double-stranded cut in the genomic DNA at the target site(s). The zygote then instigates a repair mechanism using, by an inherently error prone process, nonhomologs end joining (NHEJ) commonly leading to insertions or deletions (indels) of +1 to –1–10's of nucleotides at this region. If such indels are created within a gene, they often lead to disruptive mutations ranging from hypomorphic to true null alleles. Upon transfer of microinjected zygotes into pseudopregnant animals, offspring are born with targeted indels at efficiencies often approaching 100% (M.V. Wiles, personal observations). More often with GWAS and other disease modeling strategies, a precision nucleotide change is desired to recapitulate a human mutation – e.g., defined, exact base change(s). Such genetic editing changes can be achieved using CRISPR/Cas9 mediated homology directed repair (HDR). For this, a zygote microinjection, consisting of approximately 100 to 200 oligonucleotide donors composed of 1–50 nucleotides of novel desired base changes, flanked by sequences homologs to the targeted region, is also introduced. Such donor oligonucleotides are often perfectly incorporated into the targeted region by HDR leading to precise genetic changes, e.g., changing a single amino acid in a gene reflecting a putative disease associated change in humans.
As with all these approaches, there is always the wish for more complicated modifications, e.g., conditional KOs where the targeted region is flanked by LoxP sites precision insertion of reporter constructs, partial or even complete humanization of entire genes. All of these are possible, although as the complexity and/or scale of the required genetic modifications increase so does the difficulty of achieving success.
A key aspect of using targeted nuclease methods, which is often not fully appreciated, is that it is now relatively simple to build a mouse model on any or even multiple genetic backgrounds. This opens exploration of the impact of genetic background (variation) on the penetrance of a trait. Additionally, established models can now be sequentially modified leading to a continual refinement of models and thus more sophisticated analysis.
As with human skin diseases, there are large numbers of mouse skin diseases that occur naturally as well as those found in large-scale mutagenesis projects, be they genetically engineered (KOMP) or chemical mutagenesis methods. Mouse colonies are maintained in highly controlled environments behind barriers that minimize exposure to infectious diseases. A few examples of infectious diseases are presented. Genetic-based skin diseases, the most common types of models, can be simple single gene Mendelian traits (such as coat color) or complex polygenic traits. Those with similar phenotypes due to mutations in different genes are often caused by genes that function in the same molecular pathway. As such, rather than present an encyclopedic listing of models, representative ones that fall into known or novel and evolving pathways that illustrate the power of mouse models to unravel the complexities of skin disease in mammals, which includes humans, are presented.
Laboratory mice today are usually re-derived by cesarean section or embryo transfer, raised on microbiologically defined (specific pathogen free [SPF]) foster mothers, transferred to, and maintained in high microbiological barrier facilities to minimize exposure to opportunistic or real pathogens. In most facilities, this means no known mouse pathogens can be detected in the colony. As such, primary infections are very rare in well-run facilities making the mouse a poor model for human infectious skin diseases. Opportunistic pathogens, those considered normal commensals in a dirty environment or those carried in by animal caretakers who do not follow strict mouse handling protocols, will result in infectious disease problems, especially in mice with various types of immunodeficiencies. However, infectious disease work can be done in biocontainment facilities (ABSL2 or higher facilities). Two examples affecting the skin are presented here.
Bacterial induction of disease is minimized by autoclaving or irradiating feed, treating the water (acidification or chlorination), and maintaining a high barrier. However, not all investigators follow protocols, so accidental infection of mice with human pathogens can occur. Some bacterial infections can become quite serious in the skin of mice, for example, Staphylococcus spp. induced botryomycosis. Here, mice can present with massive abscesses often around the head from which pure cultures of the bacteria can be isolated ( Fig. 36.7 ). These infections can spread to the lungs with a miliary distribution giving the appearance of metastatic cancer. While the lesions may present as a skin lesion, entry into the body usually can be found coming through ulcerations in the epithelium surrounding the molars, which are often induced by impaction with food material and hairs (from grooming). Osteomyelitis in the maxilla spreads, resulting in prominent swelling of the face which may rupture at the skin surface. This lesion can be a major part of the phenotype of some targeted mutant mice, notably those with deficiencies in cytochrome b-245, beta polypeptide ( Cybb tm1Din ) or plasminogen activator, urokinase ( Plau tm1Mlg ), which both affect the immune system. Sentinel mice with aglobulinemia were reported to develop these lesions. Staphylococcal infections have resulted in botryomycosis in cut wounds in pathogen-free colonies.
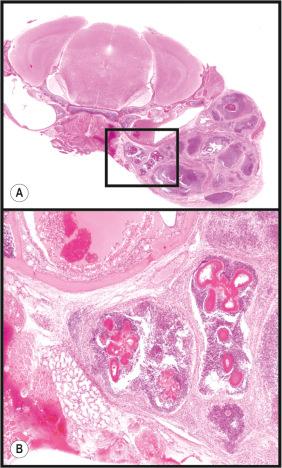
While interest in human and nonhuman mammalian papillomaviruses has been waning since the commercialization of polyvalent recombinant vaccines, discovery of a mouse papillomavirus has opened up interesting new avenues for research. Numerous rodent papillomaviruses have been found in the past 30 years including one in a wild colony of European harvest mice. However, only recently has a laboratory mouse papillomavirus been reported in immunodeficient nude mice that could be transmitted to other nude mice but only to a limited number of inbred backgrounds. It was noted that pan T-cell deficiencies in the mice were needed for these lesions to develop. A very similar viral subtype was found in the normal skin of immunocompetent mice, suggesting that this virus has adapted well to its host. In the immunodeficient strains, benign papillomas (warts) developed on the muzzle and tail skin, but invasive, poorly differentiated carcinomas resembling trichomatrixomas developed on the dorsal lumbar skin ( Fig. 36.8 ).
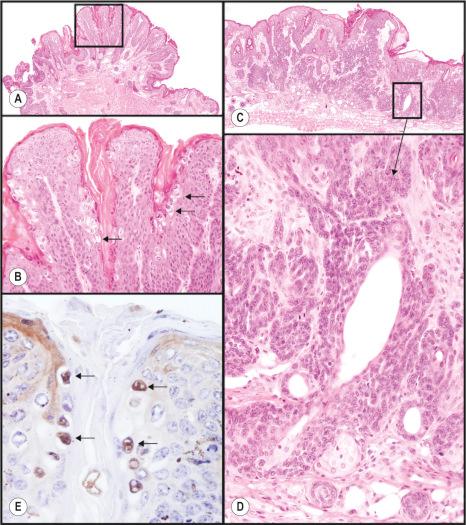
Vascular changes in the skin are commonly associated with inflammation. Mice with a chronic inflammatory skin disease that resembles psoriasis, but appears more to be a form of hypereosinophilic syndrome due to a spontaneous null mutation in the Sharpin gene, leads to the development of chronic proliferative dermatitis. Not surprisingly, there is an increase in the number and size of blood vessels in the areas of inflamed skin in these mutant mice compared to controls. These vessels can be identified easily in hematoxylin and eosin (H&E)-stained sections and confirmed using antibodies directed against smooth muscle actin for vascular smooth muscle or platelet/endothelial cell adhesion molecule 1 (PECAM1, formerly called CD31).
Lymphatics are often overlooked in the mouse because in the skin, these appear as small slits below the epidermis and parallel to the angle of the hair follicle within the dermis. Once one recognizes these structures, they are easy to identify in routine histologic sections. However, antibodies directed against lymphatic vessel endothelial hyaluronan receptor 1 (LYVE1) clearly labels the lymphatic endothelial cells. The C3H/HeJ mouse spontaneously develops alopecia areata, which has remained the model of choice for this human disease for two decades. Evaluation of skin in mice with alopecia areata as the disease progresses revealed dilation of the cutaneous lymphatics.
Genetic-based skin diseases in laboratory mice have been the most useful to study the pathogenesis in great detail of many diseases. Examples are provided below to illustrate how similar phenotypes arising in mice with known genetic mutations often fall into a common molecular pathway, and actually these features help to better define and resolve the pathway(s).
In the late 1800s, Gregor Mendel, an Augustine monk, began his work in genetics with cages of mice in his room at the Abbey in Brno, Czech Republic. Although he eventually switched to studying the inheritance of traits in plants, due to political pressures from the bishop, mice – and in particular, the coat color of mice – were used in the beginning of the field of genetics. In 1902, Lucien Cuénot, along with several other investigators, rediscovered Mendel's work and demonstrated Mendelian inheritance ratios in the coat colors of mice. The first demonstrated genetic linkage in the mouse was found between two coat color alleles, namely albino (tyrosinase [ Tyr c ]) and pink-eyed dilution (oculocutaneous albinism II [ Oca2 p ]). Over the next 80 years, mutant mice with coat color changes played a major role in constructing a linkage map of the mouse genome cementing the importance of the mouse in genetics.
In the mouse hair follicle, pigmentation follows a precise sequence of interactions between melanocytes and the dermal papilla. Hair is actively pigmented only during the anagen stage of the hair cycle. Melanin synthesis is inactive during catagen and remains so through telogen ; thus, it is important to emphasize that follicular melanogenesis is cyclic in nature.
Currently, research in the mouse has identified more genes influencing coat color than any other trait. A search of Mouse Genome Informatics ( www.informatics.jax.org ) for the phrase ‘abnormal hair/coat pigmentation’ results in 1692 genotypes affecting coat color ( http://www.informatics.jax.org ; August 2016). The most common alleles found in inbred research strains popular in research today are illustrated in Fig. 36.9 . Although coat color genetics have been studied for nearly 100 years, the great variety of alleles affecting coat color appears to be a never-ending story. Current work in this field is actively pursuing the interconnectedness of the genes to form a genetic pathway ( Fig. 36.10 ).
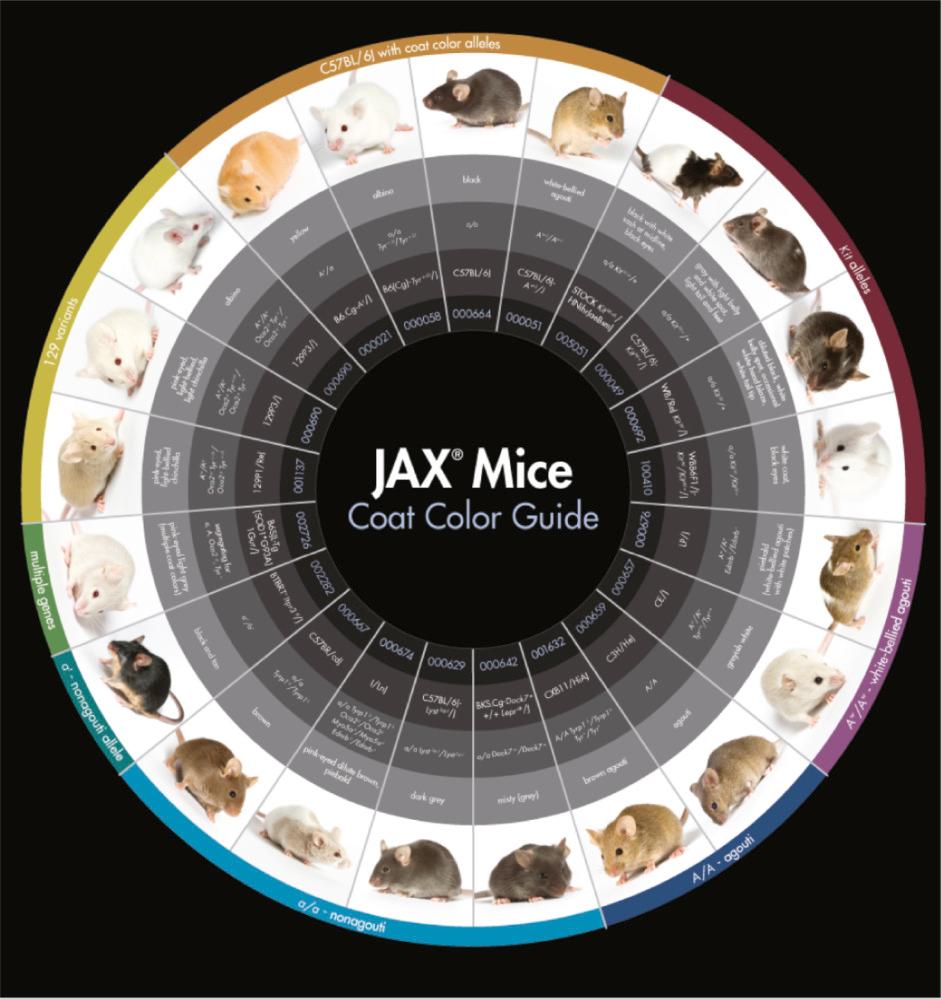
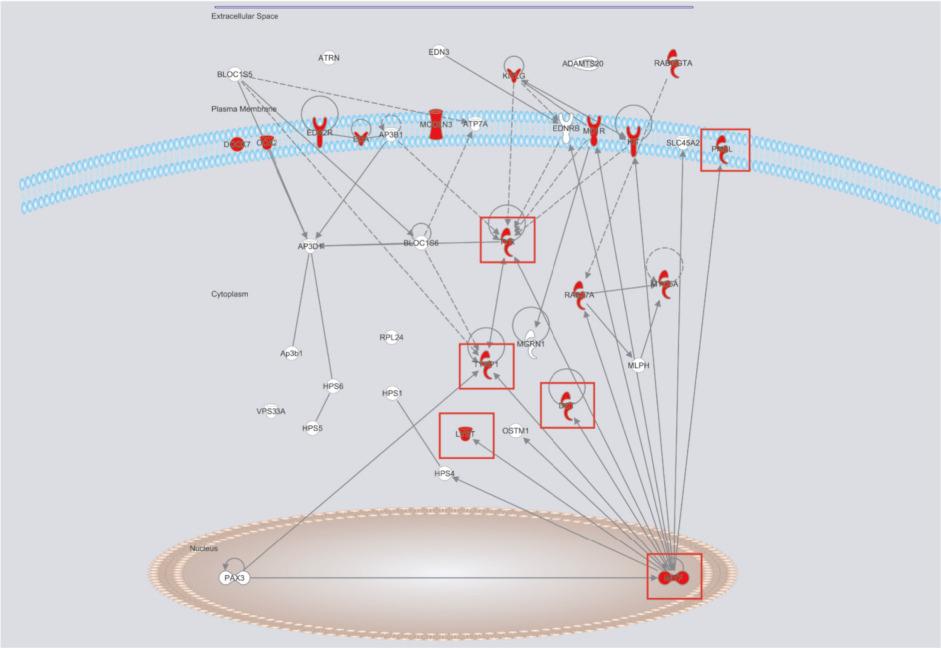
Mouse hair pigmentation is under complex genetic control as illustrated in the genetic pathway in Fig. 36.10 . The genes in this pathway have a vast array of molecular targets and functions, such as enzymes ( Tyr ), scaffolding/cytoskeletal proteins ( Pmel ), transcriptional factors ( Mitf ), as well as receptors ( Kit ) and their ligands ( Kitl ). This pathway controls hair pigmentation on the cellular (melanocyte), organ (hair follicle), and developmental (melanocyte development, migration, differentiation, proliferation, and survival) time points. These genes and pathways have homologs of great importance in human tissues and disease states.
The eumelanin (black and brown) and pheomelanin (yellow) synthesis pathways share many common steps within the melanosome of the melanocytes. Melanogenesis begins with the hydroxylation of tyrosine to l -3,4-dihydroxyphenylalanine ( l -DOPA) by the tyrosinase ( Tyr ) enzyme. l -DOPA is then oxidized to DOPAquinone, which can then be further processed through different enzymatic pathways to either eumelanin or pheomelanin. For eumelanin, DOPAquinone can spontaneously cyclize to produce DOPAchrome, which again can react in two ways. In the absence of DOPAchrome tautomerase ( Dct ) activity, the carboxyl group of DOPAchrome is lost and 5,6-dihydroxyindole (DHI) is formed. DHI is further hydroxylated by tyrosinase to become indole-5,6-quinone, that is lastly oxidated to DHI-melanin. DHI-melanin is black and insoluble. In the presence of Dct expression, DOPAchrome is tautomerized to 5,6-dihydroxyindole-2-carboxylic acid (DHICA). Tyrosinase-related protein 1 ( Tyrp1 ) catalyzes the oxidation of DHICA to indole-5,6-quinone carboxylic acid. Premelanosome protein ( Pmel ) expression functions to then polymerize indole-5,6-quinone carboxylic acid. Further oxidation forms DHICA-melanin, which is brown and poorly soluble. Synthesis of pheomelanin begins with the conjugation of DOPAquinone with cysteine or glutathione yielding cysteinylDOPA and glutathionylDOPA. CysteinylDOPA is oxidized and then spontaneously forms 1,4-benzothiazinylalanines through a tyrosinase-catalyzed oxidation and produce pheomelanin. Alternatively, DOPAquinone can be conjugated with glutathione, instead of cysteine, followed by hydrolysis of glutathionylDOPA to CysteinylDOPA.
The switch from eu- to pheomelanogenesis is regulated by a number of genes, namely pro-opiomelanocortin-alpha ( Pomc / α-Msh ), melanocortin 1 receptor ( Mc1r ), nonagouti ( A ), mahogunin, ring finger 1 ( Mgrn1 ), and attractin ( Atrn ). Eumelanin synthesis is promoted when POMC binds to the MC1R receptor, which in turn leads to the activation of the cyclic adenosine 3′–5′-monophosphate (cAMP) pathway and eumelanin synthesis. Meanwhile, pheomelanin is synthesized when the nonagouti signal protein is expressed and interacts with MC1R and ATRN to inhibit the cAMP pathway.
The agouti locus is located on chromosome 2 of the mouse genome and is associated with the nonagouti ( a ) gene. Agouti acts in the hair follicles, primarily affecting the relative amount and distribution of yellow pigment (pheomelanin) and black/brown pigment (eumelanin) in hair. Agouti mice have banded hairs whereby a band of black melanin resides at the top of the hair followed by yellow melanin in the middle and ending with black pigment near the end of the hair shaft. Currently, there are nearly 150 known variations in the agouti locus conferring a range of coat colors and pleiotropic phenotypes ( http://www.informatics.jax.org ; August 2016).
The human equivalent of the nonagouti ( a ) gene in mice is the agouti signaling protein ( ASIP ) gene located at 20q11.22. Several studies using small cohorts have found single nucleotide polymorphisms (SNPs) in and around the ASIP gene that show a weak correlation between ASIP and human skin and hair pigmentation. In addition, a melanoma risk locus was identified by three separate studies residing near ASIP . This locus increases the risk of early onset cutaneous melanoma and basal cell carcinoma.
The B or brown locus ( b ) is located on chromosome 4 of the mouse genome. This locus was identified as the tyrosinase-related protein 1 gene ( Tyrp1 ) in work during the late 1980s and early 1990s. The wildtype allele produces black eumelanin, while the recessive allele ( b ) produces brown eumelanin, making a mouse harboring the Tyrp1 b/b genotype cinnamon brown due to the occurrence of yellow-banded brown hairs. The effect on the melanosome of mice expressing the b allele versus the wildtype B allele is to change the nature of the eumelanin in the pigment granule, which is accompanied by a change in its size and shape. Brown granules are significantly smaller than black granules and display much less variation in size. The pigment granules found in the medulla of black-pigmented hairs are usually either long oval or oval in shape, while those in brown-pigmented hairs are round. Currently, there are over 60 targeted, spontaneous, and transgenic alleles of the Tyrp1 gene ( http://www.informatics.jax.org/allele/summary?markerId=MGI:98881 , MGI accessed 27 June 2016). Some of the most common alleles that can be found in laboratory mice strains include light ( B lt ), cordovan ( b c ), and white-based brown ( B w ).
The TYRP1 gene in humans resides at chromosome location 9p23 and has been linked to a number of human pigmentation disorders. Several studies have identified mutations in the TYRP1 gene that reduce the stability of the transcript and significantly inhibit its catalytic activity causing type III oculocutaneous albinism (OCA3) in either single patients or small cohorts of African Americans (106delT), southern Africans (368delA, S166X), Pakistani (R373Ter), and Asian Indian (1057delAACA) populations. Interestingly, Kenney et al. identified a homozygous change in the N terminus of TYRP1 (R93C) that causes Melanesian blond hair. Similar to the OCA3 mutation, the R93C mutation affects the stability of the TYRP1 transcript and subsequently the catalytic activity of the protein.
The c locus, better known as the albino locus ( c ), is located on chromosome 7 of the mouse and is associated with mutations in the tyrosinase ( Tyr ) gene. It is important to note that albinism is epistatic (i.e., the situation in which the effect of one gene (phenotype) is dependent on the presence of one or more modifier genes) to all other coat color determinants; thus, all mice possessing a Tyr c/c genotype will lack pigment regardless of other coat alleles. This allele is the oldest allele that has been known and documented as far back as ancient Greek and Roman mouse fanciers. Interestingly, albino animals do not lack normal melanocytes, but the Tyr c group of alleles affects the amount of tyrosinase in the melanocyte. Tyrosinase is an enzyme that catalyzes the hydroxylation of l -tyrosine to l -3,4-dihydroxyphenylalanine ( l -dopa). l -dopa is then oxidized to dopaquinone, which is a common step in both the eumelanogenic and pheomelanogenic synthesis pathways. Once l -dopa is formed, melanogenesis can proceed further through oxidation-reduction reactions and spontaneous transformations. Although Tyr encodes for tyrosinase, some albino mutations affect tyrosinase activity rather than structure, introducing several other signaling pathways and genes that may affect albinism in mice. This is illustrated in Fig. 36.10 , which shows that there are a number of genes that both directly and indirectly interact with Tyr . Normal appearing melanocytes can be found in the hair and eye, but they completely lack melanin. Pigment granules within the cells are significantly smaller and fewer than in wildtype genetic backgrounds.
The tyrosinase gene is located in the human genome at 11q14.3. As with mice, humans with mutations in the TYR gene experience pigmentation disorders. Specifically, mutations in this gene have been shown to be causative in both type IA oculocutaneous albinism (OCAIA) and type IB oculocutaneous albinism (OCAIB). OCAIA, also known as tyrosinase-negative OCA, presents with amelanotic melanocytes in the skin and subsequent loss of pigmentation in the skin, hair, and eyes. Additionally, patients may also experience ocular phenotypes reducing visual acuity including strabismus and/or nystagmus. OCAIB or tyrosinase-positive OCA arises from mutations in the TYR gene that affect the stability and catalytic activity of the tyrosinase enzyme. Thus, tyrosinase can still be detected in these patients although at a significantly reduced level. OCAIB is also known as yellow albinism. Patients with OCAIB are phenotypically similar at birth to type II oculocutaneous albinism (OCAII), yet rapidly develop normal skin pigmentation and yellow hair. Interestingly, mutations in both TYR (R402Q) and MITF (1-bp deletion in exon 8) play roles in the autosomal recessive, digenic Waardenburg syndrome type 2 with ocular albinism (WS2-OA). Patients with WS2-OA experience reduced visual acuity, photophobia, nystagmus, strabismus, and albinotic fundus with foveal hypoplasia, along with deafness.
The d locus, also known as the dilute locus ( d ), is located on chromosome 9 of the mouse genome and is associated with the myosin Va ( Myo5a ) gene. Mutations in this gene affect the clumping of melanin, thus reducing the overall absorbance of light and making the animals' color appear diluted. Melanin clumps in mice exhibiting this phenotype are much larger and more concentrated than those in black mice. These larger clumps decrease the surface area available for light absorbance by melanin. In practice, this can be seen microscopically whereby large clumps of melanin are found throughout the hair shaft. MYO5A is an actin-based motor protein that contains an N-terminal motor domain that binds to actin and a C-terminal domain that interacts with the melanosome through RAB27 (RAB27A, a member of the RAS oncogene family) and MLPH (melanophilin) (see Fig. 36.10 ). Actin-bound myosin Va functions to transport melanosomes to the cell's periphery. The melanosomes are then transported to the melanocytes dendritic extensions where they are transferred to keratinocytes.
The human equivalent of the dilute locus is the MYO5A gene located at 15q21.12. Griscelli syndrome is an autosomal recessive disorder that is characterized by pigmentary dilution. Type 1, type 2, and type 3 Griscelli syndrome are distinguished by central nervous system involvement with hypopigmentation, immunological defects with hypopigmentation, and hypopigmentation alone, respectively. Type 3 is characterized by either homozygous deletion of the MYO5A F-exon, which is a tissue-specific exon that is transcribed in melanocytes, or by a homozygous mutation in the MLPH (melanophilin) gene.
The p locus, also known as pink-eyed dilution ( p ), is located on chromosome 7 of the mouse genome and is associated with the oculocutaneous albinism II ( Oca2 ) gene. Mutations in this gene affect the size, number, and composition of melanosomes. The Oca2 gene encodes a transmembrane protein that assists in regulating the pH level within the melanosome. Wildtype Oca2 produces an intense pigmentation of the hair, but the mutant Oca2 p allele reduces the pigmentation of the coat. The eyes of Oca2 p mice resemble those of albinos, possessing a pink tint, thus the name. Oca2 p alleles reduce both black and brown pigments (eumelanin synthesis), but has only a slight influence on pheomelanin synthesis. The melanin granules within the hair shaft of Oca2 p mice are smaller than their normal counterparts. Additionally, levels of tyrosinase are reduced within melanosomes of mice possessing the mutant allele. The Oca2 gene encodes a melanosomal transmembrane protein which functions as an ion exchange protein that is similar to Na + /H + transporters. The initial step in melanogenesis requires the acidification of the melanosome, but further processing requires a rise in pH. So, one function of OCA2 is to regulate the pH of the melanosome, which in turn could regulate tyrosinase activity that is dependent on the pH of the surrounding milieu.
The p locus is located in chromosomal location 15q12-q13 in the human genome. Mutations in the human OCA2 gene have been linked to both OCAII and brown oculocutaneous albinism (BOCA) in the human population. Over 50 different mutations have been reported in the literature within the OCA2 gene, ranging from single nucleotide substitutions, to large deletions of entire exons, to complex rearrangements. Both OCAII and BOCA are tyrosinase-positive, autosomal recessive disorders whereby pigmentation is reduced in skin, hair, and eyes, and patients have characteristic vision deficits associated with albinism, although usually less severe than those with OCAI. As opposed to OCAI, patients with OCAII may experience darkening of their hair with age and may be freckled. In affected individuals with African and African American descent, patients may present with yellow hair and blue-gray iris. The BOCA variant of OCA has been observed mostly in African and African American populations and is characterized by light brown hair and skin color and gray irides.
Melanoma is a malignant disease that arises from transformation of melanocytes, the pigment producing cells of the skin and hair follicles. Spontaneous melanomas in mice are very uncommon. As a result, a variety of approaches have been undertaken in order to model the complex process of melanoma formation and progression in mice. In humans, melanocytes are predominantly located at the junction of epidermis and dermis in skin as well as in the follicular bulb and bulge and dermis, but are also present in the mucosa, the central nervous system, the eye, and inner ear. Mice also have melanocytes in similar locations; however, epidermal junctional melanocytes are mainly present in tail skin and are largely absent in trunk skin. The approaches to modeling melanoma in mice have been reviewed and are summarized below.
Early approaches utilizing the initiating carcinogen 7,12-dimethylbenz[ a ]anthracene (DMBA) were utilized to model squamous cell carcinoma and other neoplasms in mice and hamsters. It was noted that hamsters also developed highly pigmented melanocytic tumors. Many of these tumors grew slowly, and it was at times difficult to determine whether the lesions were benign or fully malignant. Ultraviolet (UV) irradiation is perhaps the most important environmental exposure leading to melanoma in humans, and it has been utilized to model skin cancer in non-genetically engineered mice. Chronic, repetitive exposure to UV light resulted in cutaneous malignant tumors, most of which represented poorly differentiated squamous cell carcinoma, occasional cutaneous sarcomas, and rarely resulted in melanoma.
The melanin pigment production pathway is unique to melanocytes, and definition of promoters of genes involved in this pathway has allowed for melanocyte-specific transgene expression. The tyrosinase ( Tyr ) promoter/enhancer construct has been utilized most often; however, promoter constructs from the dopachrome tautomerase ( Dct ) gene and microphthalmia transcription factor ( Mitf ) have also been utilized. The first transgenic mouse melanoma model utilized a Tyr promoter/enhancer construct to drive SV40 early region in melanocytes. This model resulted in highly penetrant ocular and cutaneous melanoma. Additional models involving transgenic expression of Ret , Hras , Nras , Braf , and HGF were also generated and resulted in highly penetrant melanoma formation. Several of these models utilized transgenes/oncogenes that do not appear to play a major role in human melanoma; however, collectively the models provided the opportunity to study melanoma formation and progression in genetic animal models. The models involving transgenic HGF expression are of note, as it was demonstrated that a single dose of perinatal UV exposure increased melanoma penetrance dramatically and altered the morphological features of resulting melanomas. Of note, pagetoid spread of melanoma cells is a common feature in this model and in human melanoma, but is only rarely observed in other mouse melanoma models.
The development of cre-loxP technology and two melanocyte-specific inducible cre-recombinase transgenic alleles ( Tyr :: creEr ) enabled conditional inducible gene recombination specifically in melanocytes. Spatially and temporally controlled recombination of conditional loxP -containing alleles could be induced by topical administration of 4-hydroxytamoxifen and resulting induction of melanocyte-specific cre activity. These alleles were utilized to generate a variety of genetically engineered mouse models of melanoma based on the most common genetic changes observed in human melanoma, including the BRAF V600E , NRAS Q61K , and loss of PTEN , CDKN2A , TP53 , and/or NF1. Activating mutations in the BRAF serine/threonine kinase are the most common mutations in melanoma. Two lox -based alleles were constructed in which the activated form of the BRAF kinase could be induced following exposure to cre activity. These Braf alleles were used in conjunction with Tyr::creEr alleles as the basis of the vast majority of genetically engineered mouse models. Interestingly, activation of Braf in the absence of other genetic changes induces transient proliferation of dermal melanocytes followed by growth arrest, mimicking the growth pattern of human melanocytic nevi, 80% of which have activation of Braf in the absence of other genetic changes. Additional genetic changes, including loss of the Pten , Cdkn2a , or Trp53 tumor suppressors, result in melanoma formation with complete penetrance and varying latency and multiplicity. Constitutive activation of Braf and loss of Pten results in one of the most aggressive mouse models of cancer ever developed. Roughly 10% of melanocytes undergoing these genetic changes begin dividing within 48 hours and progress on to melanoma. Induction of these genetic changes in all melanocytes in the mouse results in innumerable melanomas (> 20 000) that cover the skin of mice within 3–4 weeks. An alternative version of this model has been developed in which the cre induction agent (4-hydroxytamoxifen) is applied on the skin of mice. This is sufficient to induce local recombination of cutaneous melanocytes in the area of application and generally results in 40–100 distinct melanomas that grow confluently to form one cutaneous mass. This model has been widely utilized to evaluate the effects of therapeutic agents and modifying genetic changes. Histologically, the Braf / Pten mouse melanomas are often heavily pigmented and have unusual morphological features that somewhat resemble edematous malignant peripheral nerve sheath tumors ( Figs 36.11 and 36.12 ). Despite these unusual histologic features, there is little doubt that the tumors are melanomas, as all initial tumor formation/proliferation occurs in melanocytes, not peripheral nerve sheath cells. The Braf / Pten model illustrated a previously suspected, but unproven synergy between the mitogen-activated protein kinase (MAPK) and PI3K pathways in inducing melanoma formation. The model also demonstrated that the isolated evaluation of particular combinations of genetic changes observed in human melanoma could be precisely evaluated in genetically engineered mouse models of melanoma. An additional model of particular interest is the combination of Braf activation with Cdkn2a loss. CDKN2A is by far the most common locus for genetic changes associated with familial melanoma, accounting for approximately 40% of familial melanoma cases. Systemic activation of Braf and loss of Cdkn2a in melanocytes resulted in completely penetrant melanoma formation. However, in contrast to the Braf / Pten model, only two to four melanomas were observed per mouse. These findings underscore the role of the CDKN2A locus as a melanoma tumor suppressor, but suggest that additional stochastic changes are required in addition to Braf activation and Cdkn2a loss in order for progression to melanoma to occur. The combination of Braf activation with Trp53 loss also resulted in melanoma formation with similar latency, penetrance, and multiplicity that were observed in the Braf / Cdkn2a model, also suggesting that stochastic changes are required for full progression to melanoma in this model. Taken together, genetically engineered mouse models of melanoma involving cre-loxP technology have contributed to the validation of human candidate melanoma-relevant genetic changes and have illustrated the specific contributions of individual genetic changes.
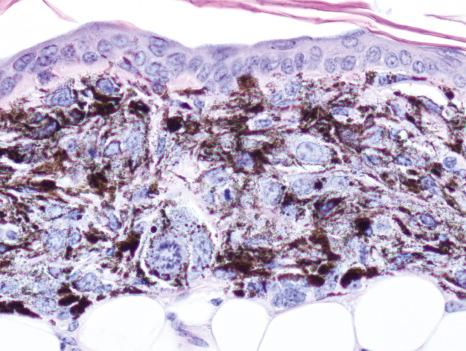
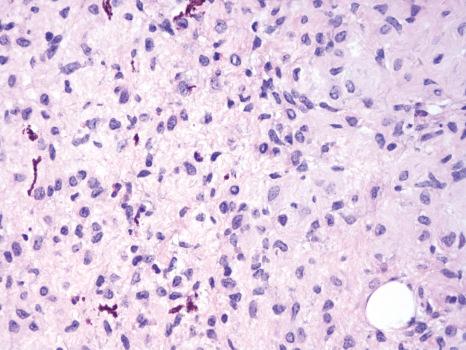
Genetically engineered mouse melanoma models are elegant and recapitulate many features of human melanoma, but are expensive and time-consuming to use for scientific experiments. An alternative is the use of mouse models that utilize melanoma cell lines that have been isolated from inbred or congenic mice that are sufficiently isogenic to a particular reference inbred genetic mouse strain to allow for tumor engraftment without graft rejection. The B16 mouse melanoma cell line was isolated in 1930 and has been widely utilized in over 17 000 publications to date. This cell is highly pigmented and has been extensively utilized to evaluate melanoma cell biology, tumor formation, and antitumor immune responses. While the B16 model has been widely utilized, the genetic drivers that resulted in tumor formation remain unknown and analogous lines with similar features or behavior have not been identified. A more recent approach has been to backcross alleles utilized to produce genetically engineered mouse models of melanoma onto a reference genetic strain (e.g., C57BL/6J), recreate the genetically engineered mouse model, induce melanoma, and derive congenic mouse melanoma cell lines with the genetic features defined by the model. The individual melanoma cell lines can then be tested for melanoma formation/engraftment into immunocompetent C57BL/6J mice. Lines that form tumors/grafts are termed syngeneic and allow for melanoma modeling with defined genetic changes in immunocompetent hosts. The dynamic interplay between the immune system and melanoma cells is of great interest given the high rates of response observed in human melanoma following immune checkpoint inhibition therapy. There is a great need for a variety of mouse models that recapitulate the immune reactions and responses to immune-based therapies observed in humans. The B16 model, however, is notoriously resistant to immune therapies and generally only responds in the context of prior vaccination protocols, which are not particularly applicable to human melanoma. A recent approach to making syngeneic mouse melanoma models more immunogenic involves the mutagenesis and single cell-derived clonal isolation of new cell lines. The theory behind this is that the immune system can recognize protein-coding differences between tumor and host if they exist. Increasing the number of protein coding changes (potential neo-epitopes) increases the chances of antitumor immune responses. This approach has been shown to be successful, allowing for the generation of models in which effective antitumor immune responses are generated, including the response to blockade of immune checkpoint inhibitors PD1 and CTLA4. Given that immune-based therapies had among the highest rates of response observed to date in a wide variety of cancers, accurate mouse models of immunogenic tumors will be of great interest as the mechanism of antitumor immune responses are determined and new combinations of immune therapies are evaluated.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here