Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Most skin surgery can be performed under local anesthesia, avoiding the risks associated with general anesthesia.
Local anesthetics reversibly interrupt propagation of nerve impulses by interfering with sodium ion influx into peripheral nerve cells.
Topical application is particularly effective for mucosal surfaces because of their enhanced absorption.
For other surfaces, intradermal or subcutaneous infiltration are the most commonly used techniques, the former being more immediate in onset and more prolonged, but also causing more tissue distortion and pain.
To anesthetize a large area of skin, a nerve block may be more appropriate, injecting a small amount at the major cutaneous nerve trunk that supplies the area, therefore avoiding the use of potentially toxic amounts of anesthetic.
Choice of anesthetic and delivery method depends on the type of surgery planned and patient characteristics.
Local adverse effects may occur, but allergic reactions are rare.
Serious systemic adverse effects can result from inadvertent intravascular injection, excess amounts, and abnormal drug metabolism; an awareness of the effects associated with the different anesthetics is important.
The expanding field of dermatologic surgery requires the proper selection and administration of anesthesia to maximize patient safety and comfort. Because it reliably provides effective anesthesia and avoids the increased risks of morbidity and mortality associated with general anesthesia, local anesthesia is preferred for most cutaneous surgical procedures.
Investigations of local anesthetic agents similar to ones used today began in the late nineteenth century. In 1860, Neiman isolated cocaine from the shrub of Erythroxylon coca and noted its numbing effect on the tongue. In 1880, Von Anrep recognized cocaine's anesthetic properties after injecting it into animals and into his own arm. In 1884, Koller, influenced by Sigmund Freud, introduced cocaine into the clinical arena when he used cocaine as a local anesthetic during surgery for a patient with glaucoma. Soon thereafter, Hall and Halsted performed the first peripheral nerve block using cocaine. However, as the use of cocaine for local anesthesia expanded, reports of its potential toxicity and addictive effects also emerged.
Safer local anesthetics were developed during the past century. In 1904, Alfred Einhorn synthesized procaine, an ester of para-aminobenzoic acid (PABA). In 1905, Braun reported the successful use of procaine with epinephrine for local anesthesia. In 1930, a more potent PABA ester, tetracaine, was introduced. Although both had utility as anesthetics, they had the tendency to produce allergic reactions. In 1943, Lofgren and Lundqvist synthesized lidocaine, an amide derivative of diethylaminoacetic acid. Its superior safety and efficacy has led to its widespread use, and lidocaine has become the prototype of local anesthetics. Subsequently, other amide derivatives have been developed.
Several local anesthetics with various methods of delivery are now available for cutaneous surgery. Appropriate, safe, and effective use of these compounds depends on choosing the correct compound, understanding its pharmacological properties, and employing the proper technique of administration. Proper use of local anesthesia maximizes patient safety, minimizes pain, and increases ease of surgical procedures.
Most local anesthetic agents have similar chemical structures, consisting of three components: an aromatic portion, an intermediate chain, and an amine portion ( Fig. 3.1 ). Modifications of any of these components can affect the pharmacological properties of the anesthetic agent. The aromatic end provides most of the lipophilic properties of the compound. It facilitates the diffusion of the anesthetic through membranes, which correlates to the potency of the anesthetic. The hydrophilic end, usually consisting of a tertiary amine, is involved in binding within the sodium channel. These two domains are linked with an intermediate chain, consisting of either an ester or amide, and having a length between 3 and 7 carbon equivalents is necessary for local anesthetic activity. Disruption of this chain initiates the drug's metabolism and allows for the reversible nature of the anesthetic.
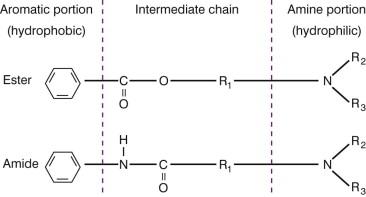
The differences in the intermediate chain linkage (ester vs amide) classify local anesthetics into two groups ( Table 3.1 ). They differ in their metabolism and potential for sensitization. Ester-type compounds tend to have a shorter duration of action because they are rapidly hydrolyzed by plasma pseudocholinesterases to form metabolites excreted by the kidneys. Individuals with decreased levels of pseudocholinesterase may be prone to the toxic effects of these agents. PABA is a major metabolic product and is responsible for the higher incidence of allergies with ester-type anesthetics. Amide derivatives are not readily hydrolyzed. They are metabolized by microsomal enzymes in the liver and excreted by the kidneys. Individuals with compromised liver function are more susceptible to the toxic effects of amide anesthetics.
| Type | Generic name | Some trade names |
|---|---|---|
| Esters | Procaine | Novocain |
| Tetracaine | Pontocaine | |
| Benzocaine | Hurricane | |
| Chloroprocaine | Nesacaine | |
| Cocaine | (None) | |
| Amides | Lidocaine | Xylocaine |
| Bupivacaine | Marcaine | |
| Mepivacaine | Carbocaine | |
| Prilocaine | Citanest |
The pharmacological properties of common ester and amide anesthetics are outlined in Tables 3.2 and 3.3 . The molecular structure and dissociation constant (pKa) of local anesthetics affect their potency and toxicity. Changes in the molecule's structure affect lipid solubility and protein binding. In general, lipid solubility determines the potency of the agent while protein binding dictates its duration of action. For example, the addition of a 4-carbon group to procaine creates tetracaine, which is more lipid soluble and potent. Highly protein-bound agents, such as bupivacaine, are tightly associated with the neural membrane, leading to a longer duration of action. The pKa influences the onset of action of local anesthetics. For the most part, the shorter-acting anesthetics tend to have a faster onset of action and less toxicity. However, peak plasma concentrations depend on various factors including the concentration of anesthesia, the duration of infiltration, the site of injection, and the rate of metabolism of the agent.
| Anesthetic | Onset (min) | pKa | Duration (min) | Maximal recommended dose (mg/kg) for adults | ||
|---|---|---|---|---|---|---|
| Without epinephrine | With epinephrine | Without epinephrine | With epinephrine | |||
| Procaine | 5 | 8.9 | 15–30 | 30–90 | 10 | 14 |
| Chloroprocaine | 5–6 | 9 | 30–60 | N/A | 10 | N/A |
| Tetracaine | 7 | 8.6 | 120–240 | 240–480 | 2 | 2 |
| Anesthetic | Onset (min) | pKa | Duration (min) | Maximal recommended dose (mg/kg) for adults | ||
|---|---|---|---|---|---|---|
| Without epinephrine | With epinephrine | Without epinephrine | With epinephrine | |||
| Lidocaine a | <1 | 7.7 | 30–120 | 60–400 | 5 | 7 |
| Bupivacaine | 2–10 | 8.1 | 120–240 | 240–480 | 2.5 | 3 |
| Mepivacaine | 3–20 | 7.6 | 30–120 | 60–400 | 6 | 8 |
| Prilocaine | 5–6 | 7.7 | 30–120 | 60–400 | 7 | 10 |
| Etidocaine | 3–5 | 7.7 | 200 | 240–360 | 4.5 | 6.5 |
| Ropivacaine | 1–15 | 8.2 | 120–360 | Not yet defined | 3.5 | Not yet defined |
| Levobupivacaine | 2–10 | 8.1 | 120–240 | Not yet defined | 2.1 | Not yet defined |
a See Table 3.11 for pediatric dosage.
The pKa of local anesthetics and the hydrogen ion concentration (pH) of the solution and tissue influence the pharmacologic activity of local anesthetics. Local anesthetics are weak bases, with a pKa between 7.7 and 9.1. They are usually prepared as solutions of hydrochloride salts with a pH of 5.0–6.0 to enhance their solubility and stability. In tissue, they exist either as an uncharged base or as a cation, with their relative proportions determined by the pKa of the anesthetic and the pH of the solution. The non-ionized base form can readily diffuse across lipid nerve sheaths and cell membranes, while the ionized form can diffuse through the extracellular space and intracellular cytoplasm. In general, a lower pKa correlates to a higher concentration of base and a faster onset of action. Alkalinization of the anesthetic solution increases the amount of base and the anesthetic's onset of action. In addition, the pH of the tissue can also affect the action of local anesthetics. Infected tissues tend to be acidic and impair the effectiveness of local anesthetics.
Local anesthetics can cross the placenta by passive diffusion; however, they are generally safe to use during pregnancy. Studies have shown no maternal or teratogenic effects from the administration of lidocaine during the first trimester of pregnancy. Lidocaine, etidocaine, and prilocaine are labeled pregnancy category B. Because of the potential of causing fetal bradycardia, bupivacaine and mepivacaine are labeled category C. Local anesthetics can be excreted in breast milk and result in toxicity to the infant if a large amount of anesthetic is used.
Local anesthetics act by reversibly interrupting the propagation of impulses and blocking nerve conduction. In the resting state, the intracellular electric potential is negative relative to the extracellular space as a result of an ionic gradient of sodium and potassium ions. This gradient is maintained by the cellular membrane, which allows free movement of potassium, and by Na + /K + adenosine triphosphatase (ATPase) pumps. During conduction of an impulse, sodium channels open and allow sodium ions to move across the membrane and generate an impulse or action potential. By interfering with the influx of sodium ions into the cells, local anesthetics prevent the depolarization of peripheral nerves and subsequent nerve conduction. The exact mechanism of action by which local anesthetics interfere with the movement of sodium ions is unclear. One postulated mechanism involves the local anesthetic binding to receptors in sodium channels, and when enough sodium channels within an axon are blocked, conduction is interrupted. The binding site of local anesthetics may be at the channel's pore or on the protein subunits within the channel.
Nerve fibers are divided into three main categories: A, B, and C fibers ( Table 3.4 ). A and B fibers are myelinated and C fibers are unmyelinated. The A fibers are the largest and are subdivided into four types: alpha, beta, gamma, and delta. The A-alpha fibers conduct motor impulses, the A-beta fibers primarily conduct light touch and pressure, the A-gamma fibers are responsible for joint proprioception, and the A-delta fibers, which are the smallest of the A fibers, conduct pain and temperature. The B fibers are preganglionic sympathetic fibers. The C fibers are the smallest and, like the A-delta fibers, conduct pain and temperature. In general, smaller myelinated fibers are easier to block than larger myelinated fibers; therefore, pain and temperature sensation may be eliminated before the loss of vibration and pressure. This translates clinically as locally anesthetized patients may not feel pain and temperature, but still feel a pressure sensation during a procedure.
| Type of nerve fiber | Myelinated | Property/Innervation | |
|---|---|---|---|
| A | alpha | Yes | Motor |
| beta | Yes | Light touch and pressure | |
| gamma | Yes | Proprioception | |
| delta | Yes | Pain and temperature | |
| B | Yes | Preganglionic sympathetic fibers | |
| C | No | Pain and temperature | |
Substances are frequently added to local anesthetics to augment analgesia and enhance the ease and safety of the surgery ( Table 3.5 ). Some of these products are commercially available pre-mixed by the manufacturer.
| Additive | Dosage | Purpose |
|---|---|---|
| Epinephrine | ≤1 : 100 000 | To decrease bleeding, prolong anesthesia, reduce anesthetic toxicity |
| Hyaluronidase | 150 units added to decrease every 30 mL of anesthetic | To facilitate drug diffusion, tissue distortion with infiltration |
| Sodium bicarbonate (8.5%) | 1 mL (1 mEq/mL) for every 10 mL of 1% lidocaine with epinephrine | To decrease pain with infiltration of acidic solution |
Most local anesthetics, except for cocaine, promote vasodilatation by relaxation of vascular smooth muscle. This results in increased bleeding at the operative site and increased diffusion of anesthetics away from the site of injection. Vasoconstrictors are commonly added to local anesthetics to decrease bleeding and thereby facilitate the ease of surgery. In addition, they retard the absorption of anesthetics, which in turn minimizes the amount of drug injected and decreases systemic toxicity. By localizing the drug to the field injected, vasoconstrictors also prolong the duration of the anesthesia. The added benefit of prolonging the duration of the anesthesia does not seem to hold true for more lipid-soluble, long-acting agents such as bupivacaine and ropivacaine, which are already highly tissue bound.
Epinephrine is the most common vasoconstrictor added to local anesthetics to enhance efficacy. Although the anesthetic may have an immediate onset of action, full vasoconstriction with epinephrine typically requires 7–15 min. Epinephrine is commercially available pre-mixed at concentrations of 1 : 100 000 and 1 : 200 000 with lidocaine. The optimal dose of epinephrine has been debated. However, for dermatologic surgery, concentrations >1 : 200 000 are probably not necessary and concentrations >1 : 100 000 are associated with an increased risk for side-effects. The maximum dose of epinephrine for local anesthesia injected in healthy individuals should generally not exceed 1 mg (100 mL of 1 : 100 000 solution) over approximately 8–10 h, which is the equivalent of 5 half-lives. However, these parameters can be significantly influenced by patient age and concomitant health issues which may affect metabolism.
Epinephrine is labeled pregnancy category C. Concerns about the safety of use during pregnancy were raised when epinephrine was shown to reduce uterine blood flow in experimental animals. Decreased placental perfusion can theoretically interfere with fetal organogenesis, particularly in the first trimester. Later in the pregnancy, decreased uterine blood flow by epinephrine absorption may induce premature labor. Given these potential risks, it is prudent to postpone non-urgent procedures requiring the use of epinephrine until after pregnancy. Some have advocated the use of dilute concentrations (e.g., 1 : 300 000) of epinephrine in necessary procedures during pregnancy.
To prevent the degradation of epinephrine in an alkaline pH, commercially prepared lidocaine with epinephrine contains acidic preservatives, such as sodium metabisulfite and citric acid. The resulting acidic solution tends to cause more pain on injection. Freshly mixed solutions of lidocaine with epinephrine result in a less acidic solution; therefore less discomfort with injection. Freshly prepared anesthetic solution having a 1 : 100 000 concentration of epinephrine can be made by adding 0.5 mL of 1 : 1000 epinephrine to 50 mL of lidocaine. Adding half as much epinephrine in this mix would give a concentration of 1 : 200 000 epinephrine.
The addition of sodium bicarbonate to commercially available lidocaine with epinephrine reduces the pain on infiltration. The pH of lidocaine is around 5.0–7.0. However, the addition of acidic preservatives lowers the pH of commercially prepared epinephrine and lidocaine solutions to around 3.3–5.5, thus causing more discomfort with injection. Buffering with 8.4% sodium bicarbonate at a ratio of 1 sodium bicarbonate to 10 epinephrine 1 : 100 000 or 1 sodium bicarbonate to 15 epinephrine 1 : 200 000 increases the pH, bringing it closer to physiologic pH, and reduces pain. Adjusting the pH of the solution can affect other pharmacologic properties of these agents. Epinephrine is chemically unstable in anesthetic solutions alkalinized by sodium bicarbonate. Neutralizing lidocaine and bupivacaine solutions containing epinephrine with bicarbonate decreases the duration of action of epinephrine and decreases the shelf-life of the mixture. In addition, alkalinization of local anesthetics allows for increased amounts of uncharged, lipid-soluble base, which more readily crosses the nerve membrane, leading to a faster onset of action. Clinically, alkalinization of mepivacaine and lidocaine for use in peripheral nerve blocks leads to more rapid nerve blockade.
Hyaluronidase is an enzyme that depolymerizes hyaluronic acid, one of the acid mucopolysaccharides present in intercellular ground substance. It is typically prepared in 150 unit vials. Its addition to local anesthetics facilitates diffusion of injectable solutions through tissue planes, thereby increasing the area of anesthesia and minimizing tissue distortion by fluid infiltration. It facilitates undermining in the subcutaneous plane by hydrodissection of fatty tissue. Clinically, hyaluronidase may be a useful adjunct in surgery in the periorbital region to minimize the number of anesthetic injection sites and potential ecchymoses. It may also be helpful in harvesting split-thickness skin grafts when wider areas of local anesthesia can be attained with minimal loss of anatomic contour. Uniform dosage recommendations for cutaneous surgery are not available, but 150 units in 20–30 mL of anesthetic have been used.
Hyaluronidase has disadvantages that limit its use in cutaneous surgery. It decreases the duration of anesthesia and potentially increases the risk of anesthetic toxicity as a result of increased absorption. Hyaluronidase contains the preservative thimerosal, which is a contact allergen. Because rare allergic reactions have been reported, preoperative skin testing has been recommended. Hyaluronidase is not recommended for tumescent liposuction because it does not augment the degree of anesthesia with the tumescent technique and increases the rate of absorption of lidocaine, and therefore the potential for systemic toxicity.
Different local anesthetics are sometimes mixed together to capitalize on the useful properties of each drug. For example, a longer-acting anesthetic with a delayed onset of action, such as bupivacaine, can be mixed with a quicker-onset anesthetic, such as lidocaine. A study that evaluated the efficacy of such a mixed solution found no difference in onset or duration of action than with bupivacaine alone. The addition of a longer-acting anesthetic into an operative site previously anesthetized with a shorter-acting anesthetic may provide more optimal anesthesia when a prolonged procedure is anticipated.
Topical application of anesthesia has been particularly effective on mucosal surfaces, but caution must be taken regarding increased systemic absorption leading to toxicity. Some products are particularly formulated for application on mucosal surfaces ( Table 3.6 ). The stratum corneum presents the major barrier to the delivery of topical anesthesia on intact skin. The development of novel delivery systems has allowed for increased penetration and greater efficacy of newer topical agents.
| Anesthetic | Trade name | Concentration (%) | Vehicle | Onset of action | Duration of action | Clinical use |
|---|---|---|---|---|---|---|
| Cocaine | 4, 10 | Solution | 1–5 min | 30–60 min | Intranasal | |
| Lidocaine | Xylocaine | 2–5 | Gel, ointment, topical and viscous solution | 1–2 min | 15–20 min | Oral mucosa |
| Benzocaine | Hurricane | 20 | Liquid, gel, spray, swab | <5 min | 15–45 min | Oral mucosa |
| Benzocaine combination | Cetacaine | a | Spray, liquid | 30 s | 30–60 min | Oral mucosa |
| Proparacaine | Alcaine | 0.5 | Solution | 20 s | 15–20 min | Ophthalmic |
| Tetracaine | Pontocaine | 0.5 | Solution | 20 s | 12–20 min | Ophthalmic |
a Cetacaine contains 14% benzocaine, 2% butyl aminobenzoate, and 2% tetracaine hydrochloride.
Cocaine is an ester anesthetic that, unlike other local anesthetics, possesses vasoconstrictive properties. Available as a 4% and 10% solution, it is primarily used for intranasal surgery. Anesthesia occurs within 5 min of application and lasts up to 30 min. The maximum recommended dose is 200 mg/kg. Potential toxicity, including hypertension, tachycardia, and arrhythmias, can result from blocking the reuptake of norepinephrine. In addition, decreased coronary blood flow can occur, leading to myocardial infarction. Dopamine reuptake blockade results in central nervous system stimulation. The risks of adverse events and the potential for abuse limit cocaine's anesthetic use over safer alternative agents.
Benzocaine is a topical ester anesthetic available as an aerosol spray, gel, ointment, or solution ranging from 5–20%. It is commonly used for achieving rapid anesthesia on mucosal surfaces. Although topical benzocaine can cause contact sensitization, it is still widely used. The 20% gel known as Hurricane gel applied with a dry gauze for 30–60 s or Hurricane aerosol spray for <2 s achieves anesthesia within 15–30 s. The anesthetic effect lasts for about 12–15 min. Available as a spray and liquid, Cetacaine is a mixture of 14% benzocaine, 2% butyl aminobenzoate, and 2% tetracaine hydrochloride that produces rapid mucosal anesthesia that lasts for approximately 30–60 min. Benzocaine-containing preparations should be avoided in infants because of the risk of methemoglobinemia.
Lidocaine, available in a 2–5% gel and topical and viscous solutions, has been used reliably for topical anesthesia on mucosal surfaces. However, these compounds are formulated in conventional vehicles that often do not provide adequate and consistent anesthesia for intact skin surfaces. A lidocaine 5% patch is available that is marketed primarily for postherpetic neuralgia. Over the past 40 years, mixtures of higher concentrations of lidocaine have been specially compounded to achieve topical anesthesia for minor surgical procedures. Topical preparations of 30% lidocaine prepared using Acid Mantle (Doak Pharmacal Co Inc, Westbury, NY) or Velvachol (Novartis, East Hanover, NJ) as a vehicle result in hydrophilic mixtures that hydrate the stratum corneum and facilitate penetration of lidocaine through the stratum corneum. Over the past decade or so, topical anesthetics in more sophisticated vehicles have become commercially available that allow for better efficacy in reducing pain during superficial cutaneous surgery ( Table 3.7 ).
| Anesthetic | Ingredients | Vehicle | Onset of action (min) |
|---|---|---|---|
| EMLA a | 2.5% lidocaine (lignocaine) with 2.5% prilocaine | Oil in water | 60–120 |
| ELA-Max (now LMX-4) a , b | 4% lidocaine | Liposomal | 30–60 |
| ELA-Max 5 (now LMX-5) a , b | 5% lidocaine | Liposomal | 30–60 |
| Topicaine a , b | 4% lidocaine | Microemulsion | 30–60 |
| Tetracaine c | 4% tetracaine | Lecithin gel | 60–90 |
| Betacaine-LA c | Lidocaine, prilocaine, dibucaine | Vaseline ointment | 60–90 |
A widely used agent, EMLA (eutectic mixture of local anesthesia) cream, is a 5% eutectic mixture composed of 2.5 mg/mL lidocaine and 2.5 mg/mL prilocaine in an oil-in-water emulsion cream. A eutectic mixture is a formulation that melts at a lower temperature than any of its individual components. EMLA's formulation contains emulsifiers that enhance skin penetration and increase the anesthetic concentration to 80% in the oil droplets, while maintaining a low overall concentration of 5%, thereby minimizing the risk of systemic toxicity. Several clinical trials have shown its efficacy in alleviating pain during various dermatologic procedures, including laser surgery, chemical peels, harvesting split-thickness skin grafts, skin biopsies, and curettage and electrosurgery.
EMLA is available as a cream and a patch. It has a child-resistant closure that complies with the Poison Prevention Packaging Act issued by the US Consumer Product Safety Commission requiring products containing over 5 mg of lidocaine in a single package to be child resistant. EMLA is available as a 30 g tube in retail pharmacies and a 5 g tube in the hospital setting for inpatient use. EMLA is also packaged as an anesthetic disc, which contains 1 g of EMLA emulsion, with an active contact surface area approximately 10 cm 2 . Generally, a 60-min application period under an occlusive dressing, such as Tegaderm (3M Healthcare, St Paul, MN) or Saran Wrap (Dow Chemical Company, Midland, Michigan), is needed before the procedure; however, this may vary depending on the location of the treatment. Effective anesthesia after 25 min of EMLA application to the face and after 5–15 min on mucosal surfaces has been reported. Increased duration of application over 2 h has been shown to correspond to enhanced depth of analgesia. The depth of analgesia after 60 min is 3.0 mm and after 120 min, 5.0 mm. Because of the risk of methemoglobinemia associated with prilocaine, EMLA should be used with caution in infants. Alkaline injury to the cornea has been seen with EMLA, so the use of EMLA close to the eyes should be avoided.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here