Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Patients with HIV/AIDS may develop cutaneous manifestations that reflect their immune-compromised state and can be grouped into infections (viral, bacterial, fungal, and parasitic/ectoparasitic), inflammatory, neoplastic, or other conditions.
Syphilis, caused by Treponema pallidum , is a sexually transmitted disease that has experienced a resurgence with the HIV epidemic, and it is classically divided into four stages: primary, secondary, latent, and tertiary, with each stage potentially having cutaneous manifestations.
Causes of sexually transmitted infectious genital ulcers include herpes simplex, syphilis, chancroid, and, less commonly, granuloma inguinale or lymphogranuloma venereum. Appropriate diagnostic testing should be performed in order to distinguish these entities and institute appropriate therapy. One should always bear in mind that all genital ulcers are not infectious, and a differential diagnosis should always be considered.
The current Centers for Disease Control and Prevention (CDC) recommendations for treatment have been updated in 2010 and 2012 due to changes in antibiotic susceptibilities, and these are summarized and discussed.
The focus of this chapter is diseases that are sexually transmitted with dermatologic manifestations. A few disorders, e.g., mollusca contagiosa, condylomata acuminata, and scabies, are mentioned but not discussed in detail. Discussions of herpes simplex, herpes zoster, and reactive arthritis, formally referred to as Reiter’s syndrome, are found in Chapter 7, Chapter 31 .
The acquired immunodeficiency syndrome (AIDS) is due to infection with one of two retroviruses, HIV-1 (more virulent and the cause in the majority of patients) or HIV-2. In the early 1980s, several of the initial descriptions of patients with AIDS came from dermatologists as they noted the appearance of cutaneous Kaposi’s sarcoma in a new patient population. The affected individuals were not elderly men of Mediterranean or Ashkenazi Jewish descent as expected, but rather young men who had had sex with other men, and, who often had multiple partners. Over the years, additional high-risk populations were identified, including hemophiliacs who had received contaminated factor VIII concentrates, injection-drug users, and commercial sex workers and their sexual partners. In developing countries, where there is a larger burden of HIV infection, the predominant mode of transmission is heterosexual.
Sub-Saharan Africa is the most affected region, with 24.7 million people living with HIV in 2013. Also, sub-Saharan Africa accounts for almost 70% of the global total of new HIV infections. In the United States and Western Europe, the annual incidence of AIDS has reached a plateau. Public education, routine testing of pregnant women followed by antiretroviral treatment of those who are HIV-positive, and highly active antiretroviral combination therapy (HAART) have all played a role in this stabilization. With HAART therapy, however, has come a novel set of cutaneous side effects discussed below.
The majority of patients in the United States with HIV are unaware of their infection, and are responsible for the majority of new infections; many expert consensus statements recommend more widespread, or universal, screening for HIV. Dermatologists should have a low threshold to offer HIV testing in their practice.
In this section, the cutaneous manifestations of HIV infection and AIDS will be divided into infectious, inflammatory, neoplastic, and miscellaneous.
The cutaneous manifestations of HIV infection and AIDS that are infectious in nature can be categorized into four major groups: viral, bacterial, fungal, and parasitic/ectoparasitic.
Acute retroviral syndrome occurs approximately 2 to 4 weeks after acute HIV infection. This process is a self-limiting clinical syndrome of varying severity. It presents with “flu-like” symptoms such as fever, fatigue, weight loss, headache, myalgias, pharyngitis, lymphadenopathy, in addition to a morbilliform (measles-like) cutaneous eruption and mucosal ulcerations. Cutaneous immune responses to the HIV virus dissemination are possibly the cause of the maculopapular rash. Because the symptoms of the acute retroviral syndrome are nonspecific and anti-HIV antibodies are usually negative at this stage, the diagnosis is established by detecting plasma viral RNA (usually >5 × 10 4 copies/mL, and as high as 1 × 10 6 copies/mL) and/or p24 antigen. The viremia is accompanied by a drop in the circulating CD4+ T-lymphocyte count and a rise in the CD8+ T-lymphocyte count.
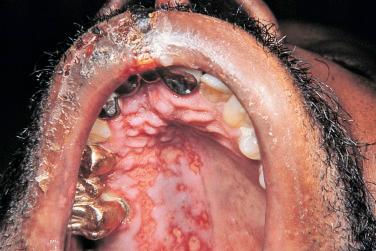
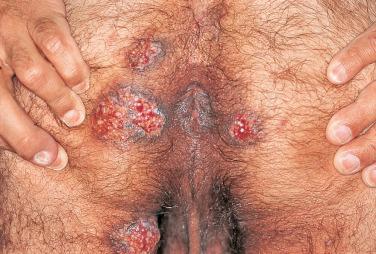
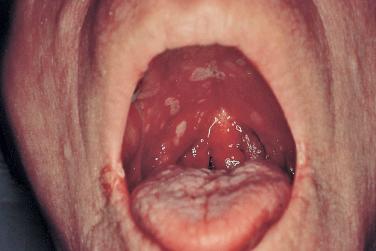
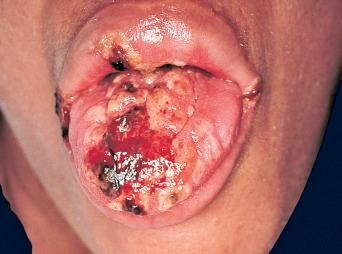
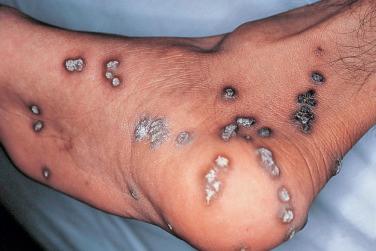
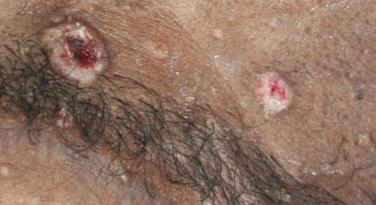
Mucocutaneous infections due to herpes simplex virus (HSV)-1, HSV-2, and the varicella-zoster virus (VZV) are commonly seen in association with HIV infection and AIDS; the typical clinical presentations of these three viruses are reviewed in Chapter 31 . Because herpes zoster (uni- or multidermatomal) can serve as one of the presenting signs of HIV infection ( Fig. 35-1 ), this diagnosis should prompt a discussion of risk factors. In contrast to the self-limiting episodes of herpes zoster or orogenital herpes simplex that occur in immunocompetent hosts, patients with AIDS can have lesions that last for months until appropriate therapy is instituted. More recently, the development of herpes zoster or severe recurrent HSV infection has been described in the setting of immune reconstitution inflammatory syndrome (IRIS) on commencement of HAART. Chronic perianal ulcers due to HSV are sometimes misdiagnosed as decubitus ulcers ( Fig. 35-2 ) and chronic oral ulcers as aphthous stomatitis ( Fig. 35-3 ), until a direct immunofluorescence antibody assay, viral culture, or polymerase chain reaction (PCR) of scrapings from the ulcer edge points to the correct diagnosis. One clue to the diagnosis of HSV as the etiologic agent is scalloping of the border of the ulcer and preceding vesicles ( Fig. 35-4 ). In patients with AIDS, chronic dermatomal and disseminated VZV lesions can become verrucous ( Fig. 35-5 ) and the clinical diagnosis requires a high index of suspicion. For patients who are immunocompromised, including those with AIDS, antiviral therapy should be continued until there is complete clinical resolution. Hospitalization or intravenous therapy may be necessary in some cases.
When lesions of HSV or VZV fail to improve despite appropriate therapy (systemic acyclovir, famciclovir, or valacyclovir), the possibility of an acyclovir-resistant strain needs to be excluded, especially when patients have been receiving chronic suppressive therapy with oral acyclovir. Intravenous foscarnet (which does not require phosphorylation to interact with viral DNA polymerase) is the recommended treatment for acyclovir-resistant strains. However, there are reports of patients developing foscarnet-resistant strains of HSV owing to mutations in the DNA polymerase gene. An alternative treatment is topical (1% gel), intralesional, or intravenous cidofovir.
Administration of the varicella vaccine to prevent primary infection is also an important strategy to protect children and adults who do not have a prior history of varicella. The vaccine can be given to HIV-infected patients who have CD4 T-lymphocyte counts of >200 cells/μL despite the theoretical risk of live-virus vaccination in this population. Some clinicians choose to offer the zoster vaccine to HIV-infected persons who are >50 years of age and have good HIV control and CD4 counts of >200 cells/μL; however, zoster vaccination should be avoided in patients with CD4 counts of <200 cells/μL. (Note that the vaccine used to prevent reactivated VZV disease [Zostavax] contains 14 times the amount of virus as the vaccine to prevent primary infection.) If an HIV-infected adult develops a postvaccination varicella-like cutaneous eruption a prompt evaluation should be made and consideration should be given to starting antiviral therapy.
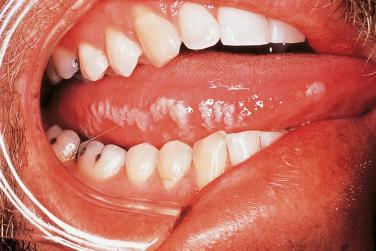
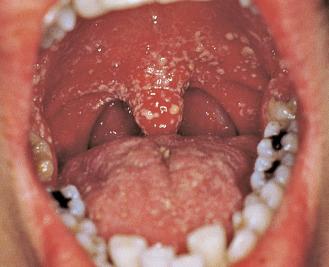
Infections with two other members of the herpes family, cytomegalovirus (CMV) and Epstein–Barr virus (EBV), are seen in HIV-infected individuals. The most common EBV-induced mucocutaneous disorder is oral hairy leukoplakia, which is characterized by filiform white plaques and papules, primarily on the lateral aspects of the tongue ( Fig. 35-6 ). These lesions may have a somewhat corrugated appearance, and improvement is seen in the setting of HAART therapy.
Although the disorder most commonly associated with CMV is retinitis, this herpes virus can lead to gastrointestinal (GI) ulcers and, occasionally, orogenital or perianal ulcers as well as morbilliform eruptions. The detection (via specific immunohistochemical stains) of intranuclear CMV inclusions within dermal endothelial cells usually proves to be a more sensitive assay than viral cultures. In mucocutaneous ulcers, CMV often “coexists” with other viruses such as HSV or VZV, and as a result its true pathogenicity has been questioned.
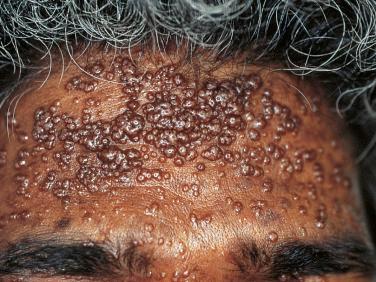
Because condylomata acuminata and mollusca contagiosa (MC)—cutaneous viral infections due to human papilloma viruses (HPV) and poxvirus, respectively—can be sexually transmitted, HIV-infected patients are at increased risk for both. Because of the coexistent immunosuppression, their clinical manifestations frequently become quite exaggerated; for example, the individual lesions of MC often coalesce into broad-based plaques and may become so large as to be referred to as “giant.” As expected, the condylomata involve the anogenital region, but in addition to the genitals, the most common site of involvement for MC is the face ( Fig. 35-7 ). Even banal warts (verruca vulgaris) or flat warts can become widely distributed, as in organ transplant patients ( Fig. 35-8 ), and often persist despite HAART.
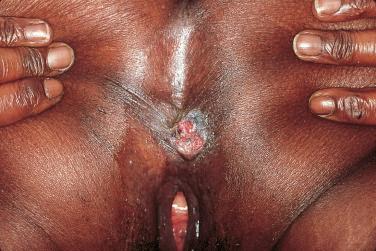
In women, infections with certain subtypes of HPV, e.g., 16 and 18, are associated with the development of cervical cancer, and this risk increases in the setting of HIV infection. These same HPV strains also increase the risk of vulvar, penile, and anal carcinoma ( Fig. 35-9 ). HIV-infected men who have sex with men, with high-risk HPV infection, are at greatest risk for anal cancer. Serial anal examinations in combination with anal cytology have been advocated for screening such patients, as has histologic examination of any suspicious lesion.
MC and HPV infections may prove recalcitrant to standard destructive therapies (e.g., cryotherapy, curettage, etc.), but improvement of MC has been observed after the institution of HAART. Both MC and condylomata acuminata may respond to topical imiquimod or cidofovir; occasionally, intralesional or intravenous cidofovir is administered for extensive confluent MC or condyloma. In one study of HIV-positive men who have sex with men and anal intraepithelial neoplasia, imiquimod cream (for perianal disease), and suppositories (for intra-anal disease) led to clinical and histologic clearing in 75% of those who used the medication as directed (three times per week for 16 weeks).
Cutaneous bacterial infections that are seen in association with HIV infection and AIDS can be subdivided into a few major categories: soft tissue infections and folliculitis due primarily to Gram-positive cocci; bacillary angiomatosis; sexually transmitted diseases (STDs) (see below); and mycobacterial and atypical mycobacterial infections. Because HIV is often acquired via sexual contact, patients who are HIV-positive need to be screened for other STDs, and vice versa. In addition, the presence of erosions and ulcerations in the anogenital region due to bacterial diseases, such as syphilis, or viral diseases, such as HSV, can increase the transmission rate of HIV.
Soft tissue bacterial infections in patients with HIV are often due to Staphylococcus aureus or Streptococcus spp., and their clinical presentations range from folliculitis and abscesses to cellulitis and necrotizing fasciitis ( Fig. 35-10 ). When S . aureus is identified as the etiology of a cutaneous infection, the possibility of nasal carriage needs to be considered as well as the possibility of meticillin-resistant S. aureus . The cutaneous manifestations of atypical mycobacterial infections are also protean and can vary from subtle areas of erythema to necrotic ulcers. Several species of atypical mycobacteria have been isolated from skin lesions in patients with HIV/AIDS, which may be isolated or disseminated, including Mycobacterium haemophilum , M . fortuitum , M. malmoense , and M . avium intracellulare . HIV-infected patients are also at risk for developing cutaneous tuberculosis, including disseminated miliary tuberculosis. Multidrug treatment regimens for cutaneous mycobacterial infections are the same as for systemic disease.
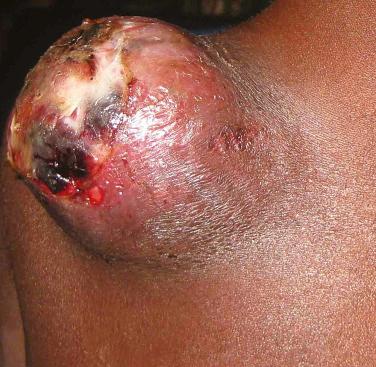
Bacillary angiomatosis is a bacterial infection whose diagnosis warrants the exclusion of an underlying HIV infection or immunosuppression. Only occasionally is it seen in normal hosts. The microorganisms responsible for the appearance of discrete proliferations of cutaneous blood vessels are Bartonella henselae and B . quintana ; the former is also the etiologic agent of cat scratch disease. The characteristic skin lesion is a red to violet papule that resembles a “hemangioma,” pyogenic granuloma, or a lesion of Kaposi’s sarcoma ( Fig. 35-11 ). Additional sites of involvement include the subcutis, with the formation of nodules, the liver (peliosis hepatitis), lymph nodes, bones, and heart (endocarditis). The diagnosis is established by identifying the bacteria in Warthin–Starry-stained histologic sections or by PCR-based analysis of biopsy specimens. Treatment consists primarily of first- and second-generation macrolides.
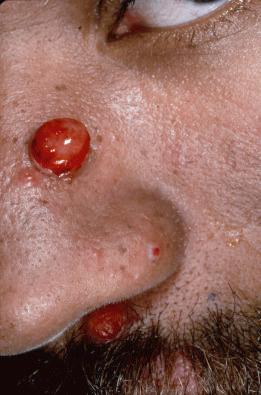
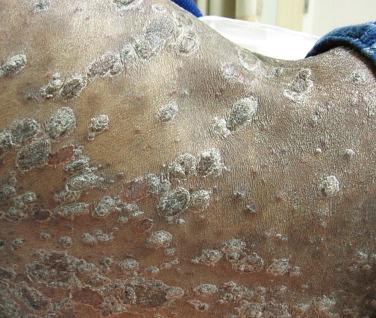
Certain fungal infections, such as oral candidiasis (thrush), can serve as presenting signs of HIV infection ( Fig. 35-12 ), whereas others, such as cryptococcosis, are seen late in the course of the disease. HIV-infected patients are at increased risk for the development of superficial mucocutaneous fungal infections due to Candida spp. and dermatophytes, as well as systemic infections due to Cryptococcus neoformans , Candida spp., and dimorphic fungi (e.g., Histoplasma capsulatum , Coccidioides immitis , Penicillium marneffei ). The cutaneous lesions of cryptococcosis and dimorphic fungal infections are often a mixture of indurated papules, plaques, and ulcerated lesions, and they sometimes resemble the dome-shaped papules of MC ( Fig. 35-13 ). Examination of a potassium hydroxide preparation of dermal scrapings or a periodic acid–Schiff- or silver-stained histologic section allows a presumptive diagnosis, and a simultaneous tissue fungal culture provides a definitive diagnosis. Cryoptococcal cutaneous infections are often a sign of disseminated disease, and should prompt thorough multisystem evaluation including a lumbar puncture.
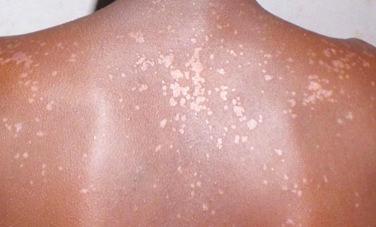
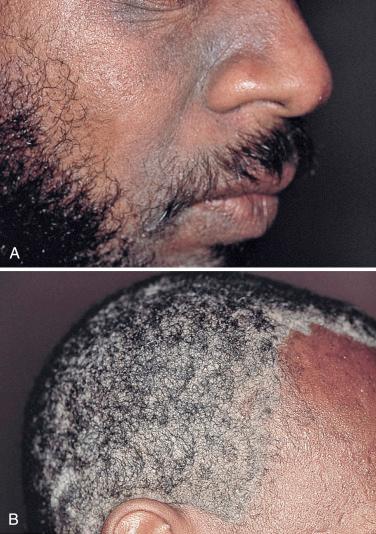
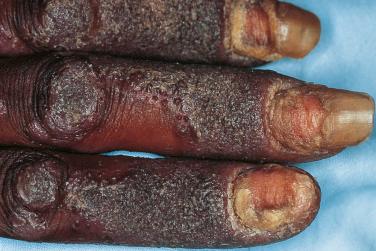
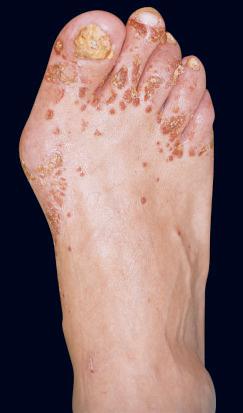
Infections with tinea (dermatophytoses) affect three keratin-containing structures, the skin, hair, and nails. HIV-infected patients are not only prone to the more common forms of tinea, e.g., tinea pedis, tinea manuum (hand), tinea unguium (nails), and tinea corporis ( Fig. 35-14 ), but they often have more severe or widespread lesions. These patients also develop an unusual form of tinea unguium known as proximal subungual onychomycosis. In the more common form of tinea unguium (distal subungual onychomycosis), the most pronounced dystrophic changes, e.g., thickening and yellow discoloration, are seen in the distal portions of the fingernails or toenails, whereas in the proximal form there is a white discoloration that first appears in the proximal portion of the nail. This proximal subungual form has also been observed in other immunocompromised patients, such as those who have received organ transplants.
DNA analyses have led to a reclassification of Pneumocystis jiroveci ( carinii ) as a fungal species. Pneumonia is the most common clinical presentation of P . jiroveci infection, but rarely cutaneous lesions do develop, especially in the external auditory canal and nares.
One of the more common ectoparasitic infections in patients who have AIDS is scabies, in particular a form characterized by thick scale-crusts and numerous mites of Sarcoptes scabiei var. hominis ; this is sometimes referred to as crusted or Norwegian scabies. Unlike the form of scabies seen in immunocompetent hosts, in which each person is infested with a few dozen mites, these patients have hundreds to thousands of mites and are therefore highly contagious. The thick scale is one of the explanations for the difficulty in eradicating crusted scabies with topical scabicides, such as 5% permethrin cream. One of the off-label uses of oral ivermectin is the treatment of such patients, who often require combination regimens with prolonged and repeat treatments.
HIV-infected patients frequently develop folliculitis, and its etiology can vary from S . aureus and herpes simplex to Malassezia spp., dimorphic fungi, and the Demodex mite. Although the latter ectoparasite is part of the normal inhabitants of the hair follicle, its presumed pathogenetic role is based upon the numerous Demodex mites found in the expressed follicular contents from AIDS patients with folliculitis in whom there was no other explanation for the folliculitis.
Patients with AIDS can also present with cutaneous lesions due to systemic infections with parasites such as Toxoplasma gondii (primarily the central nervous system [CNS]), Acanthamoeba spp. (primarily CNS and sinuses), Strongyloides stercoralis (primarily GI tract and lung), Leishmania spp. (primarily bone marrow), and microsporidia (primarily GI tract). Although these secondary cutaneous lesions are rather unusual and do not have unique clinical presentations, the diagnosis can be made fairly easily by identification of the responsible organisms in biopsy specimens.
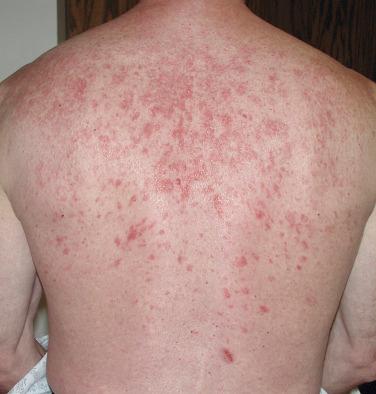
There are several inflammatory skin disorders that can serve as the initial clinical manifestation of HIV infection, in particular seborrheic dermatitis, psoriasis, and reactive arthritis (the disorder previously referred to as Reiter’s syndrome—see Chapter 7 ) ( Figs. 35-15 to 35-17 ). For some patients, this represents new-onset disease, whereas in others there is a flare of a preexisting dermatosis. In this particular clinical setting, seborrheic dermatitis, especially of the face, may be moderate to severe in intensity and the psoriasis rather extensive. Many years ago, an increased number of Malassezia organisms (yeasts that are part of the normal flora) were observed in HIV-infected patients with seborrheic dermatitis, and this observation contributed to the subsequent use of antifungal creams and shampoos for the treatment of this disease, both in immunocompromised and immunocompetent hosts.
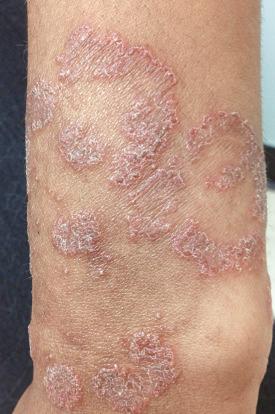
People with advanced HIV often exhibit psoriasis ( Fig. 35-18 ). The prevalence of psoriasis in HIV-positive individuals is comparable to that of the general population. However, psoriasis tends to be more severe in people infected with HIV. A much higher prevalence of psoriatic arthritis occurs in HIV-positive individuals with psoriasis than in those without the infection. A distinguishing hallmark feature of HIV-associated psoriasis compared with classic psoriasis is that several morphologic types can coexist in the same patient. The main postulated mechanisms of pathogenesis are a T-cell imbalance characterized by decreased CD4 T cells and a relative increase in CD8 memory T cells, a cytokine milieu predominantly mediated by interferon-γ, superantigen stimulation of autoimmunity by HIV, or other viral and bacterial infections and HLA Cw0602 status. The management of moderate and severe HIV-associated psoriasis is challenging and the risk-to-benefit ratio specific to these patients needs to be taken into account when selecting therapies such as phototherapy, acitretin, methotrexate, cyclosporine, hydroxyurea, TNF inhibitors, e.g., infliximab. Patients typically improve with early placement on highly active antiretroviral therapy.
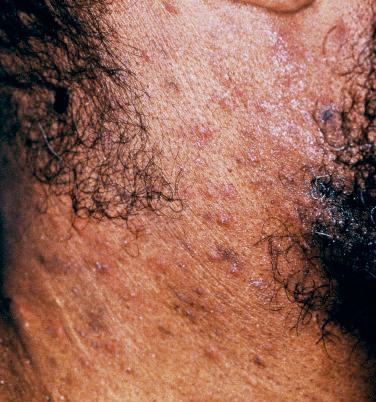
Pruritus is a frequent complaint in patients with AIDS, and secondary lesions that simply represent chronic rubbing, e.g., lichen simplex chronicus and prurigo nodularis, can be seen admixed with more specific primary lesions. Once scabies, drug eruptions, specific dermatoses such as eczematous eruptions, arthropod bite reactions, and “systemic” causes of pruritus (e.g., cholestasis, renal failure, lymphoma, parasitic infections) are excluded, the differential diagnosis includes xerosis (see below), folliculitis, eosinophilic folliculitis, and the pruritic papular eruption of HIV/AIDS. Eosinophilic folliculitis is characterized by pruritic edematous perifollicular papules, primarily on the upper trunk and in the head and neck region ( Fig. 35-19 ), and it is usually associated with advanced disease. In biopsy specimens, eosinophils are found within hair follicles. The skin-colored papules of the pruritic papular eruption of HIV/AIDS tend to favor the extremities, but can also involve the trunk; one hypothesis is that the eruption represents an exaggerated response to arthropod bites or other allergens. Both disorders may improve following UVB phototherapy, as well as with immune reconstitution.
Patients who are HIV-positive have an increased incidence of cutaneous drug eruptions, usually morbilliform or urticarial, but others include photolichenoid, erythrodermic, drug hypersensitivity syndrome, pigmentary changes, injection site reaction, retinoid-like reaction, or the spectrum of Stevens–Johnson syndrome/toxic epidermal necrolysis. This proclivity for developing allergic drug reactions (approximately 10 times greater than in the general population) became apparent when antibiotic prophylaxis became commonplace, especially the use of oral trimethoprim–sulfamethoxazole against P. jiroveci ( carinii ) pneumonia. Possible explanations for this phenomenon include the immune dysregulation that accompanies HIV infection, or a predisposition due to coexisting viral infections, e.g., CMV or EBV. As the name implies, photolichenoid eruptions favor sun-exposed areas, are often associated with sulfa-containing medications, and may present as areas of hyperpigmentation. However, idiopathic photosensitivity is also seen in patients with AIDS.
Lastly, cutaneous small-vessel vasculitis and erythema elevatum diutinum, a form of chronic cutaneous vasculitis often associated with streptococcal infections, are also thought to be more prevalent in HIV-infected patients. An idiopathic disorder that presents with skin-colored papules is granuloma annulare ( Fig. 35-20 ), and in HIV-infected patients the generalized form is seen more often than the localized form (the opposite is true in immunocompetent hosts); occasionally, even oral lesions are noted.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here